DOI:
10.1039/C4RA12487A
(Paper)
RSC Adv., 2015,
5, 9255-9260
A study on the effects of K2ZrF6 as an additive on the microstructure and hydrogen storage properties of MgH2
Received
16th October 2014
, Accepted 9th December 2014
First published on 9th December 2014
Abstract
In this work, the hydrogenation properties of MgH2-doped K2ZrF6 with X wt% (X = 5, 10, 15 and 20) have been investigated for the first time. Analysis indicated that MgH2 doped with 10 wt% K2ZrF6 is the best composite for the improvement of the hydrogen storage properties of MgH2. The results showed that the onset desorption temperature after the addition of 10 wt% K2ZrF6 was 250 °C, which experienced the reduction of 100 °C and 200 °C from 350 °C and 450 °C for as-milled and as-received MgH2, respectively. The re/dehydrogenation kinetics also significantly improved compared to the un-doped MgH2. The results of the Arrhenius plot exhibited that the activation energy for the hydrogen desorption of MgH2 was reduced from 164 kJ mol−1 to 80 kJ mol−1 after the addition of 10 wt% K2ZrF6. Moreover, the X-ray diffraction spectra displayed the formation of new phases of KH and ZrH2 by the doping of K2ZrF6 with MgH2 after the dehydrogenation and rehydrogenation processes. These two compounds are believed to act as the active species and play a catalytic role in improving the hydrogen storage properties. It is, therefore, concluded that this newly developed catalytic doping system works well in improving the hydrogen sorption properties of MgH2.
1. Introduction
Even though hydrogen is an ideal energy carrier, there are still extensive technological challenges that limit its uses as a fuel. Hydrogen can be used as a fuel in PEMFC (proton exchange membrane fuel cell), which is a very promising power source for electric vehicles (EV).1 According to the U.S. DOE's 2010 target, large volumetric (>62 kg m−3) and gravimetric (>6.5 wt%) densities are strictly needed for on-board hydrogen storage in fuel cell vehicles. To accomplish the DOE's targets, a high-performance hydrogen storage system is desired. High-pressure and cryogenic hydrogen storage systems are impractical for vehicular applications due to safety concerns and volumetric constraints. In the recent years, attention has been focused on solid hydrogen storage materials due to their significant advantages related to safety, energy efficiency and cost effectiveness.2 To date, many types of solid-state hydrogen storage materials, such as metal hydrides,3–6 complex hydrides,7–13 carbon materials,14,15 and metal–organics frameworks (MOFs),16,17 have been explored. Among all of these materials, metal hydrides have attracted intensive research interest because of their high hydrogen storage capacities and moderate working temperatures. Among the various metal hydrides, MgH2 is highly recommended due to its high hydrogen capacity with a theoretical value of 7.6 wt%, high reversibility, low cost18–20 and reserves in the earth's crust.21 Therefore, MgH2 is viewed as one of the most promising hydrogen storage materials. Unfortunately, the practical application of MgH2 is greatly limited by its slow kinetics and high operation temperature.22 Its working temperature is above 300 °C, and the de/re-hydrogenation kinetics are slow; these issues limit its practical application for on-board hydrogen storage. To overcome these problems, considerable effort has been made in the past decade to improve its hydrogen storage properties, such as combining with other materials (destabilization of the system),23–30 improving the surface and kinetics by ball milling,31 and doping with catalysts.32–36 Among them, the introduction of catalysts or additives into MgH2 by ball milling method has shown a significant effect on the hydrogen storage properties of MgH2.
Another promising additive for MgH2 is K2ZrF6. It is believed that upon heating, K2ZrF6 and MgH2 will react by an in situ reaction to form active species that enhance the hydrogen sorption properties of MgH2. Xiao et al.37 demonstrated the superior effects of KH for improving the dehydrogenation properties of NaAlH4. It has also been confirmed that ZrH2 can provide favourable effects for enhancing the dehydrogenation properties of MgH2.38 Therefore, it is reasonable to believe that K2ZrF6 shows great potential as an additive to advance the hydrogen storage properties of MgH2 by combining together these in situ metal hydride positive factors.
In this work, K2ZrF6 was introduced to examine its improvement of the hydrogen storage performance of MgH2 obtained by ball milling. To the best of the our knowledge, no studies have reported the effects of K2ZrF6 on the microstructure and hydrogen storage properties of MgH2. The hydrogen storage properties and reaction mechanisms of MgH2 and K2ZrF6 were investigated in experiments using a Sievert-type pressure-composition-temperature (PCT) apparatus, scanning electron microscopy (SEM) and X-ray diffraction (XRD). The possible catalysis mechanism supported by the results of the experiment was then discussed.
2. Experimental details
Pure MgH2 (hydrogen storage grade with 98% purity) and K2ZrF6 (97% purity) were purchased from Sigma Aldrich and were used as received without further purification. Approximately 200 mg of MgH2 doped with 20 mg of K2ZrF6 was loaded into a sealed stainless steel vial together with four hardened stainless steel balls in a MBraun Unilab glove box in an argon atmosphere. For simplicity, the mixture of MgH2 with 10 wt% of K2ZrF6 will be referred to as MgH2-10 wt% K2ZrF6 sample. The sample was then milled in a planetary ball mill (NQM-0.4) for 1 h, first by milling for 0.25 h, resting for 2 min, and then repeating the milling for another 2 cycles in a different direction at the rate of 400 rpm. The as-received MgH2 was also prepared under the same conditions for comparison purposes. The de/rehydrogenation experiments were performed in a Sievert-type pressure-composition-temperature (PCT) apparatus (Advanced Materials Corporation). The composite was loaded into a sample vessel in the glove box. For the temperature-programmed-desorption (TPD) experiment, all the composites were heated in a vacuum chamber, and the amount of desorbed hydrogen was measured to determine the lowest decomposition temperature. The heating rate for the TPD experiment was 5 °C min−1, and the samples were heated from room temperature to 450 °C. The re/dehydrogenation kinetics measurements were performed at the desired temperatures with initial hydrogen pressures of 33.00 atm and 1.00 atm, respectively.
XRD analysis was performed using a Rigaku MiniFlex X-ray diffractometer with Cu Kα radiation scans carried out over diffraction angles from 20° to 80° at a speed of 2° min−1. Prior to the measurement, a small amount of sample was uniformly placed on the sample holder, which was wrapped with plastic wrap to avoid oxidation. Moreover, the surface morphology of the composite was observed using a scanning electron microscope (SEM; JEOL JSM-6360LA) by setting the samples on carbon tape and then coating the samples with gold spray under vacuum.
3. Results and discussion
3.1 Dehydrogenation temperature
Fig. 1 shows the TPD performances for the dehydrogenation of as-received MgH2, as-milled MgH2 and MgH2 doped with 5 wt%, 10 wt%, 15 wt% and 20 wt% K2ZrF6. From the TPD patterns, it is evident that doping with a 5 wt% to 20 wt% of K2ZrF6 to MgH2 resulted in a drop of the onset desorption temperature compared to un-doped MgH2. The as-received MgH2 started to dehydrogenate H2 at a temperature of 450 °C, and the total dehydrogenation capacity was 7.5 wt% H2. The onset desorption temperature of MgH2 after milling slightly dropped to about 350 °C, showing that the process of milling also affected the onset desorption temperature.31 After milling, the curve shows that there was no reduction in the hydrogen desorption capacity of MgH2. The addition of K2ZrF6 greatly improved the onset desorption temperature of MgH2. MgH2 doped with 5 wt% K2ZrF6 started to decompose at about 260 °C with a total dehydrogenation capacity of 6.6 wt% H2. For the 10 wt% doped sample, the dehydrogenation occurred at 250 °C, which represents a decrease in the onset desorption temperature of about 100 °C from 350 °C for as-milled MgH2 with a total dehydrogenation capacity of 6.6 wt% H2, which is the same as the hydrogen desorption capacity of the 5 wt% doped sample. Further increasing the doping amounts to 15 wt% and 20 wt% reduced the desorption onset temperatures to about 280 °C and 290 °C, but the amount of hydrogen released slightly dropped to about 5.6 wt% and 5.3 wt% H2, respectively. This phenomena may be due to the excessive catalytic effects brought about by the relatively high levels of added K2ZrF6, and this result is almost the same as that reported in our previous paper.19
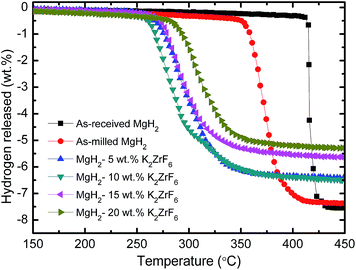 |
| | Fig. 1 TPD patterns for the dehydrogenation of as-received MgH2, as-milled MgH2 and MgH2 doped with 5 wt%, 10 wt%, 15 wt% and 20 wt% K2ZrF6. | |
3.2 Re/dehydrogenation kinetics
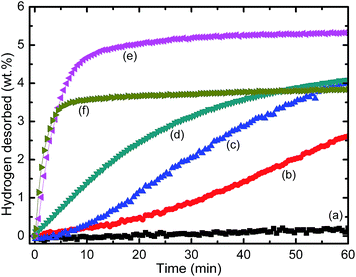
Fig. 2 depicts the hydrogen absorption rate of MgH2 after ball milling for an hour, and samples of MgH2 doped with K2ZrF6, which were also milled for an hour at 300 °C under 33.0 atm. The results were compared, and it was found that MgH2 that has been ball milled for an hour can absorb about 3.3 wt% of H2 in 5 min, and approximately 4.0 wt% of H2 in 60 min. On the other hand, after adding 10 wt% K2ZrF6 to MgH2, it was found that a slight improvement was attained. About 3.5 wt% of H2 was absorbed in 5 min and approximately 4.1 wt% of H2 can be absorbed in 60 min. Moreover, for the 5 wt%, 15 wt% and 20 wt% K2ZrF6-doped MgH2, the hydrogen absorbed as much as 3.2 wt%, 3.3 wt% and 3.0 wt% H2 within 5 min. Fig. 3 displays that the MgH2 samples doped with 5 wt%, 10 wt%, 15 wt% and 20 wt% K2ZrF6 released about 3.7 wt%, 4.1 wt%, 4.0 wt% and 3.6 wt% hydrogen at 300 °C in 60 min, respectively, whereas the un-doped MgH2 sample desorbed only 0.3 wt% hydrogen in the same period of time. The doped composites displayed an enhancement in terms of desorption kinetics. The results of the desorption onset temperature and isothermal re/dehydrogenation kinetics indicated that the sample of 10 wt% K2ZrF6 shows the best performance in improving the hydrogen storage properties of MgH2, and thus can be considered to be the best sample of MgH2 doped K2ZrF6. Therefore, the MgH2 doped with 10 wt% K2ZrF6 was chosen for further analysis.
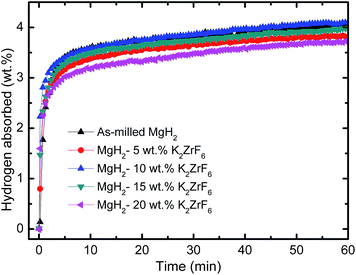 |
| | Fig. 2 Isothermal absorption kinetics measurements of as-milled MgH2 and MgH2 doped with 5 wt%, 10 wt%, 15 wt% and 20 wt% K2ZrF6 at 300 °C. | |
 |
| | Fig. 3 Isothermal desorption kinetics curves for as-milled MgH2 and MgH2 doped with 5 wt%, 10 wt%, 15 wt% and 20 wt% K2ZrF6 at 300 °C. | |
To further analyse the hydrogen desorption kinetics of the MgH2-10 wt% K2ZrF6 sample, isothermal dehydrogenation measurements were performed at different temperatures. Fig. 4 compares the isothermal dehydrogenation kinetics between the as-milled MgH2 and MgH2-10 wt% K2ZrF6 sample at 300 °C, 320 °C and 350 °C. The dehydrogenation kinetics test was carried out after the rehydrogenation kinetics process under 33.0 atm of H2 at 300 °C, 320 °C and 350 °C. The result was as expected, as the dehydrogenation rate for the as-milled MgH2 was lower than that for the doped MgH2 at 350 °C (Fig. 4c and f). After the addition of K2ZrF6, the desorption kinetics increased significantly, as the 10 wt% K2ZrF6-doped MgH2 released 4.5 wt% of H2 within 10 min at 350 °C. Under the same conditions, it was shown that the amount of hydrogen released from the as-milled MgH2 was less than 0.5 wt%, and only 2 wt% even after 20 min. Further investigation of the 10 wt% K2ZrF6-doped MgH2 at 300 °C and 320 °C showed similar results (Fig. 4((a, d) and (b, e))), where doped MgH2 revealed better desorption kinetics than the un-doped MgH2. This improvement is due to the energy barrier, where the activation energy for the release of hydrogen from MgH2 is reduced by the addition of K2ZrF6. The activation energy can be calculated using the Arrhenius equation as follows:
| |
k = k0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−EA/RT) exp(−EA/RT)
| (1) |
where
k is the rate of hydrogenation,
k0 is a temperature independent coefficient,
EA is the apparent activation energy for hydride decomposition,
R is the gas constant and
T is the absolute temperature. By plotting the graph of ln(
k)
vs. 1/
T, as indicated in
Fig. 5, the apparent activation energy,
EA, for H
2 to be released from the as-milled and K
2ZrF
6-doped MgH
2 was revealed. From these calculations, the activation energies for the as-milled MgH
2 and K
2ZrF
6-doped MgH
2 were 80 kJ mol
−1 and 164 kJ mol
−1, respectively. There was an improvement of 84 kJ mol
−1, according to the result. Based on the lowering of the activation energy, the decomposition of MgH
2 was substantially improved.
 |
| | Fig. 4 Isothermal dehydrogenation curves of as-milled MgH2 and 10 wt% K2ZrF6-doped MgH2 at 300 °C (a, d), 320 °C (b, e) and 350 °C (c, f). | |
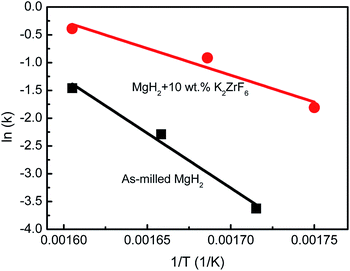 |
| | Fig. 5 Arrhenius plots of ln(k) vs. 1/T for as-milled MgH2 and MgH2 + 10 wt% K2ZrF6. | |
The cycling performance of MgH2 doped with K2ZrF6 composite was further investigated. As shown in Fig. 6, a temperature of 300 °C was applied in life cycle studies of the rehydrogenation kinetics of the MgH2 + 10 wt% K2ZrF6 composite. The absorption kinetics continued to be good, even after the 10th cycle, with a hydrogen capacity of about 3.9 wt%. As shown in Fig. 7, the life cycle desorption kinetics of MgH2 + 10 wt% K2ZrF6 at 300 °C showed almost no decrease in hydrogen storage capacity within 60 min even after 10 cycles, maintaining a capacity of about 4.1 wt%. The result shows that K2ZrF6 is a good additive for the cycle life of MgH2.
 |
| | Fig. 6 Isothermal rehydrogenation kinetics of the MgH2 + 10 wt% K2ZrF6 composite in the 1st, 5th and 10th cycles. | |
 |
| | Fig. 7 Isothermal dehydrogenation kinetics of the MgH2 + 10 wt% K2ZrF6 composite in the 1st, 5th and 10th cycles. | |
3.3 Scanning electron microscopy
Fig. 8 illustrates the grain size of the as-received MgH2, as-milled MgH2, and K2ZrF6-doped MgH2. The SEM examination revealed that the grain size before ball milling (Fig. 8(a)) was the largest compared to the as-milled MgH2 (Fig. 8(b)). Moreover, as can be seen from Fig. 8(c), the grain size of K2ZrF6-doped MgH2 was smaller than the as-milled MgH2. The significant decrease in the grain size of doped MgH2 increased the surface area of contact of MgH2. As the surface area of contact increases, the rate of reaction at which MgH2 releases and absorbs H2 will increase. Mustafa and Ismail39 reported that a smaller particle size will improve the hydrogen re/desorption, because it reduces the diffusion length of hydrogen and makes the particles' reactive surfaces larger. Hence, the activation energy and re/desorption will be improved.
 |
| | Fig. 8 SEM images for as-received MgH2 (a), as-milled MgH2 (b) and MgH2 doped with 10 wt% K2ZrF6 (c). | |
3.4 X-ray diffraction
To determine the chemical composition of K2ZrF6-doped MgH2 after milling, XRD scans were obtained after the dehydrogenation and re-hydrogenation processes. Fig. 9 depicts the XRD patterns of MgH2-10 wt% K2ZrF6 after 1 h ball milling, after dehydrogenation at 450 °C and after rehydrogenation at 300 °C. From the pattern, after 1 h ball milling (Fig. 9(a)), only MgH2 peaks were detected and no K2ZrF6-containing phases existed. The absence of the peaks of K2ZrF6 might be due to its transformation into an amorphous state during the milling, or the amount of K2ZrF6 might be too little to be detected by the matrix of XRD. After dehydrogenation at 450 °C (Fig. 9(b)), distinct peaks of Mg were present, which indicates that the dehydrogenation process was complete. Minor peaks of KH and ZrH2 were present after the dehydrogenation process, as they are the active species in the combination of MgH2 and K2ZrF6 after ball milling or heating processes. The spectra of re-hydrogenated K2ZrF6-doped MgH2 (Fig. 9(c)) represent major peaks of Mg, where peaks of MgH2 were less and the active species of KH and ZrH2 were still present. From these results, it can be determined that MgH2 is not fully reversible because the peaks of Mg are still present in the rehydrogenation phase.
 |
| | Fig. 9 X-ray diffraction patterns of MgH2-10 wt% K2ZrF6 (a) after milling, (b) after dehydrogenation, and (c) after rehydrogenation. | |
In order to verify the phase structure of the doped samples in more detail, XRD measurements were obtained using the 20 wt% K2ZrF6 added MgH2 sample. After increasing the amount of catalyst to 20 wt%, the peaks for the KH and ZrH2 species increased, as shown in Fig. 10. Due to its amorphous state, the peaks for the MgF2 species could not be observed in the XRD pattern. This finding corroborates with the study by Sun et al.40 that Ag–MgF2 cermet films consist of a mainly amorphous MgF2 matrix with embedded fcc-Ag nanocrystalline grains. Moreover, the absence of the Mg peak indicated that by increasing the amount of catalyst to 20 wt%, MgH2 can be entirely reversible, as can be seen in Fig. 9. The reaction between MgH2 and K2ZrF6 in the dehydrogenation stage can be represented by the following equation:
| | |
6MgH2 + 2K2ZrF6 → 6MgF2 + 4KH + 2ZrH2 + 2H2
| (2) |
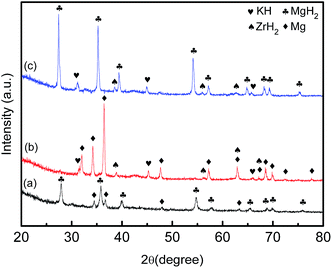 |
| | Fig. 10 X-ray diffraction patterns of MgH2-20 wt% K2ZrF6 (a) after milling, (b) after dehydrogenation and (c) after rehydrogenation. | |
The values for the standard Gibbs free energy, ΔG°f, of MgH2, MgF2, KH and ZrH2 are −35.98, −1071.10, −34.06, and −128.87 kJ mol−1,41 respectively. In addition, the standard Gibbs free energy, ΔG°f of K2ZrF6 is −2456 kJ mol−1.42 Therefore, the total change in ΔG° will be −1691.26 kJ mol−1 for MgH2. This result confirms the possibility of reaction (2) from the thermodynamic potentials. The standard-state free energy of reaction, ΔG°, can be calculated as follows:
| | |
ΔG° = ∑ΔG°f products − ∑ΔG°f reactants
| (3) |
The results of this study prove that the formation of active species of KH and ZrH2 during the de/rehydrogenation process from the reaction of MgH2 and K2ZrF6 may play an important role to enhance the sorption properties of MgH2. This discovery verifies the work by Xiao et al.37 that the addition of KH can effectively improve the dehydrogenation properties of the second step reaction of a NaAlH4 system. In addition to that, Luo et al.43 verified that the addition of a catalytic amount (<4 mol%) of KH significantly increased the hydrogen absorption rate of the desorbed material, and had a less significant effect on the kinetics of hydrogen desorption, as also shown by Wang et al.44 On the other hand, many findings have exposed that mixing MgH2 with ZrH2 improves hydrogenation behaviour.45,46 For example, Kyoi et al.38 claimed that mixing MgH2 with ZrH2 to form face centred cubic (FCC) hydrides reduced hydrogen-desorption temperatures by 170 K compared to MgH2. In addition, among the unstable reaction products, ZrH2 has a very stable phase as reported by Jat et al.,45 as well as excellent stability under irradiation conditions.46 Therefore, it is sensible to assume that this newly developed product of KH and ZrH2 acts as a real catalyst to enhance the hydrogen sorption properties of MgH2 by serving as an active site for the nucleation and growth of dehydrogenation products.
4. Conclusion
In summary, the improvement of the hydrogen desorption properties of MgH2 have been verified after adding the catalyst K2ZrF6. Among the different amounts of K2ZrF6 added, 10 wt% K2ZrF6 shows the best performance in improving the hydrogenation properties of MgH2. The TPD result exhibited that the MgH2-10 wt% K2ZrF6 sample started to release H2 at about 250 °C, where the onset desorption temperature was reduced by approximately 100 °C and 200 °C compared to the as-milled and as-received MgH2, respectively. The dehydrogenation and rehydrogenation kinetics of the doped MgH2 also improved significantly compared to the undoped MgH2. From the Arrhenius plot, the results showed that the activation energy of MgH2 after doping with 10 wt% of K2ZrF6 was reduced by approximately 84 kJ mol−1. Nevertheless, the hydrogen release capacity decreased slightly due to the dead weight of K2ZrF6 that contained no hydrogen. In addition, new phases of KH and ZrH2 formed by the doping of K2ZrF6 with MgH2 during the heating process are believed to act as the active species. From all the obtained results, it can be concluded that the products formed from the addition of K2ZrF6 play a catalytic role in improving the hydrogen storage properties of MgH2.
Acknowledgements
The authors would like to acknowledge Universiti Malaysia Terengganu for providing the facilities to carry out this project. This work was financially supported by the Fundamental Research Grant Scheme (FRGS 59295). F. A. Halim Yap and N. S. Mustafa are thankful to the Ministry of Education Malaysia for the MyBrain15 scholarship.
References
- J. G. Carton and A. G. Olabi, Energy, 2010, 35, 2796–2806 CrossRef CAS PubMed.
- S. Srinivasa Murthy and E. Anil Kumar, Appl. Therm. Eng., 2014, 72, 176–189I CrossRef CAS PubMed.
- N. S. Mustafa and M. Ismail, Int. J. Hydrogen Energy, 2014, 39, 15563–15569 CrossRef CAS PubMed.
- F. Cuevas, D. Korablov and M. Latroche, Phys. Chem. Chem. Phys., 2012, 14, 1200–1211 RSC.
- L. Mooij and B. Dam, Phys. Chem. Chem. Phys., 2013, 15, 11501–11510 RSC.
- X. B. Yu, Y. H. Guo, Z. X. Yang, Z. P. Guo, H. K. Liu and S. X. Dou, Scr. Mater., 2009, 61, 469–472 CrossRef CAS PubMed.
- M. Ismail, Y. Zhao, X. B. Yu and S. X. Dou, Int. J. Hydrogen Energy, 2010, 35, 2361–2367 CrossRef CAS PubMed.
- X. B. Yu, Y. H. Guo, D. L. Sun, Z. X. Yang, A. Ranjbar, Z. P. Guo, H. K. Liu and S. X. Dou, J. Phys. Chem. C, 2010, 114, 4733–4737 CAS.
- M. Ismail, Y. Zhao, X. B. Yu, A. Ranjbar and S. X. Dou, Int. J. Hydrogen Energy, 2011, 36, 3593–3599 CrossRef CAS PubMed.
- X. B. Yu, D. M. Grant and G. S. Walker, J. Phys. Chem. C, 2008, 112, 11059–11062 CAS.
- M. Ismail, Y. Zhao, X. B. Yu, I. P. Nevirkovets and S. X. Dou, Int. J. Hydrogen Energy, 2011, 36, 8327–8334 CrossRef CAS PubMed.
- Y. Zhang, Q. Tian, J. Zhang, S.-S. Liu and L.-X. Sun, J. Phys. Chem. C, 2009, 113, 18424–18430 CAS.
- M. Ismail, Int. J. Hydrogen Energy, 2014, 39, 8340–8346 CrossRef CAS PubMed.
- P. Kowalczyk, R. Holyst, M. Terrones and H. Terrones, Phys. Chem. Chem. Phys., 2007, 9, 1786–1792 RSC.
- Y. Zhang, W.-S. Zhang, A.-Q. Wang, S. Li-Xian, M.-Q. Fan, H.-L. Chu, J.-C. Sun and T. Zhang, Int. J. Hydrogen Energy, 2007, 32, 3976–3980 CrossRef CAS PubMed.
- M. Hirscher and B. Panella, J. Alloys Compd., 2005, 404–406, 399–401 CrossRef CAS PubMed.
- C.-Y. Wang, C.-S. Tsao, M.-S. Yu, P.-Y. Liao, T.-Y. Chung, H.-C. Wu, M. A. Miller and Y.-R. Tzeng, J. Alloys Compd., 2010, 492, 88–94 CrossRef CAS PubMed.
- A. Ranjbar, Z. P. Guo, X. B. Yu, D. Wexler, A. Calka, C. J. Kim and H. K. Liu, Mater. Chem. Phys., 2009, 114, 168–172 CrossRef CAS PubMed.
- M. Ismail, Int. J. Hydrogen Energy, 2014, 39, 2567–2574 CrossRef CAS PubMed.
- M. Y. Song, Y. J. Kwak, H.-S. Shin, S. H. Lee and B.-G. Kim, Int. J. Hydrogen Energy, 2013, 38, 1910–1917 CrossRef CAS PubMed.
- S.-S. Liu, L.-X. Sun, J. Zhang, Y. Zhang, F. Xu, Y.-H. Xing, F. Li, J. Zhao, Y. Du, W.-Y. Hu and H.-Q. Deng, Int. J. Hydrogen Energy, 2010, 35, 8122–8129 CrossRef CAS PubMed.
- X. B. Yu, Z. X. Yang, H. K. Liu, D. M. Grant and G. S. Walker, Int. J. Hydrogen Energy, 2010, 35, 6338–6344 CrossRef CAS PubMed.
- S. Kato, A. Borgschulte, M. Bielmann and A. Zuttel, Phys. Chem. Chem. Phys., 2012, 14, 8360–8368 RSC.
- M. Ismail, Y. Zhao, X. B. Yu, J. F. Mao and S. X. Dou, Int. J. Hydrogen Energy, 2011, 36, 9045–9050 CrossRef CAS PubMed.
- Y. Jia, C. Sun, L. Cheng, M. Abdul Wahab, J. Cui, J. Zou, M. Zhu and X. Yao, Phys. Chem. Chem. Phys., 2013, 15, 5814–5820 RSC.
- M. Ismail, Y. Zhao and S. X. Dou, Int. J. Hydrogen Energy, 2013, 38, 1478–1483 CrossRef CAS PubMed.
- G. S. Walker, M. Abbas, D. M. Grant and C. Udeh, Chem. Commun., 2011, 47, 8001–8003 RSC.
- M. Ismail, Y. Zhao, X. B. Yu and S. X. Dou, RSC Adv., 2011, 1, 408–414 RSC.
- Y. Zhang, Q.-F. Tian, S.-S. Liu and L.-X. Sun, J. Power Sources, 2008, 185, 1514–1518 CrossRef CAS PubMed.
- M. Ismail, Y. Zhao, X. B. Yu and S. X. Dou, Int. J. Hydrogen Energy, 2012, 37, 8395–8401 CrossRef CAS PubMed.
- R. A. Varin, M. Jang, T. Czujko and Z. S. Wronski, J. Alloys Compd., 2010, 493, L29–L32 CrossRef CAS PubMed.
- H. Shao, M. Felderhoff and F. Schüth, Int. J. Hydrogen Energy, 2011, 36, 10828–10833 CrossRef CAS PubMed.
- B. Zhang and Y. Wu, Int. J. Hydrogen Energy, 2014, 39, 13603–13608 CrossRef CAS PubMed.
- H. Leng, Y. Pan, Q. Li and K.-C. Chou, Int. J. Hydrogen Energy, 2014, 39, 13622–13627 CrossRef CAS PubMed.
- M. Ismail, N. Juahir and N. S. Mustafa, J. Phys. Chem. C, 2014, 118, 18878–18883 CAS.
- M. Ismail, Y. Zhao, X. B. Yu and S. X. Dou, Energy Educ. Sci. Technol., Part A, 2012, 30(spec. iss. 1), 107–122 Search PubMed.
- X. Xiao, S. Wang, X. Fan, C. Xu, J. Sun, Q. Wang and L. Chen, Int. J. Hydrogen Energy, 2014, 39, 6577–6587 CrossRef CAS PubMed.
- D. Kyoi, T. Sakai, N. Kitamura, A. Ueda and S. Tanase, J. Alloys Compd., 2008, 463, 311–316 CrossRef CAS PubMed.
- N. S. Mustafa and M. Ismail, Int. J. Hydrogen Energy, 2014, 39, 15563–15569 CrossRef CAS PubMed.
- Z.-Q. Sun, D.-M. Sun, A.-X. Li and Z.-Y. Xu, Chin. Phys. Lett., 1999, 16, 389 CrossRef CAS.
- http://www.chemistry-reference.com/StandardThermodynamicValuesat25°C.
- M. V. Glazoff, Thermodynamic Assessment of Hot Corrosion Mechanisms of Superalloys Hastelloy N and Haynes 242 in Eutectic Mixture of Molten Salts KF and ZrF4, INL/EXT-12-24617, Revision 0, 2012, p. 4 Search PubMed.
- W. Luo, V. Stavila and L. E. Klebanoff, Int. J. Hydrogen Energy, 2012, 37, 6646–6652 CrossRef CAS PubMed.
- J. Wang, T. Liu, G. Wu, W. Li, Y. Liu, C. M. Araújo, R. H. Scheicher, A. Blomqvist, R. Ahuja, Z. Xiong, P. Yang, M. Gao, H. Pan and P. Chen, Angew. Chem., Int. Ed., 2009, 48, 5828–5832 CrossRef CAS PubMed.
- R. A. Jat, S. C. Parida, R. Agarwal and K. L. Ramakumar, Int. J. Hydrogen Energy, 2014, 39, 14868–14873 CrossRef CAS PubMed.
- D. Chattaraj, S. C. Parida, S. Dash and C. Majumder, Int. J. Hydrogen Energy, 2014, 39, 9681–9689 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−EA/RT)
exp(−EA/RT)







