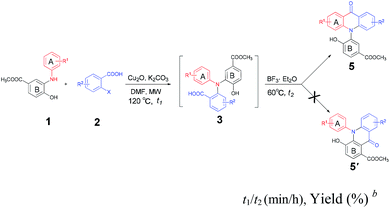
A biomass-involved strategy for the synthesis of N-arylated dibenzo[b,e][1,4]oxazepin-11(5H)-ones, acridones, 7,12-dihydrodibenzo[b,e][1,4]oxazocin-6H-ones and dibenzo[b,f]azepin-10(11H)-ones†
Ensheng Zhangabd,
Xuejing Zhanga,
Wen Weib,
Dejian Wangbd,
Yuchen Caic,
Tianlong Xubd,
Ming Yana and
Yong Zou*abd
aSchool of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou, 510006, P. R. China. E-mail: zou_jinan@163.com; Fax: +86 20 39943499; Tel: +86 20 39943499
bGuangzhou Institute of Chemistry, Chinese Academy of Science, Guangzhou, 510650, P. R. China
cSun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, 510060, P. R. China
dUniversity of Chinese Academy of Sciences, Beijing, 100039, P. R. China
First published on 1st December 2014
Abstract
An efficient method for the assembly of novel and diversified benzo-fused N-heterocycles mediated by the biomass-derived N-arylated 2-aminophenol (1) has been established. Four kinds of benzo-fused N-heterocycles with six- to eight-membered ring systems, including N-arylated dibenzo[b,e][1,4]oxazepin-11(5H)-ones (4), acridones (5), 7,12-dihydrodibenzo[b,e][1,4]oxazocin-6H-ones (6) and dibenzo[b,f]azepin-10(11H)-ones (7) have been efficiently synthesized in good to excellent yields, respectively. This process is comprised of the N-arylation reaction between N-arylated 2-aminophenols (1) and 2-halogenated benzoic or phenylacetic acids to afford a series of mutli-functionalized triarylamines (3), followed by chemo- and regio-selective intramolecular lactonization or acylation under mild conditions.
Introduction
Benzo-fused N-heterocycles, especially those with six- to eight-membered systems, such as dibenzoazepines and acridones represent an intriguing class of heterocycles prevalent in various pharmaceuticals, bioactive compounds and nature products.1 For instance, the most frequently used antiepileptic drugs carbamazepine2 and its analogue oxcarbazepine3 as well as the histone deacetylase inhibitor4 (I) are all characterized by the dibenzoazepine core structure. The cytotoxic agent chloro acridone5 (II) and the pyranoacridone alkaloid acronycine6 (III) also possess an acridone scaffold (Fig. 1). To the best of our knowledge, although great efforts have been made for the construction of these benzo-fused six- to eight-membered N-heterocycles,7 the structural diversity of these compounds is still limited. Moreover, no protocol for the construction of N-aryl-dibenzo[b,e][1,4]oxazepin-11(5H)-ones (4), N-aryl-7,12-dihydrodibenzo[b,e][1,4]oxazocin-6(H)-ones (6) as well as N-aryl-dibenzo[b,f]azepin-10(11H)-ones (7) has been reported thus far (Scheme 1). In addition, only a few approaches for access to N-aryl acridones have been documented. Ground-breaking works leading to N-aryl acridones reported by Cheng and Deng, respectively, involved the reaction of copper-catalyzed intramolecular oxidative C–H functionalization and C–N bond formation of phenyl-(2-phenylaminophenyl)methanone.8 Another method for the synthesis of N-aryl acridones includes an intermolecular insertion reaction of benzyne with amides or β-lactams, followed by stepwise intramolecular transformations.9 Although each of these approaches represents a workable access to N-aryl acridones, novel and efficient methods are still needed. In view of the above, a practical approach that is facile, efficient and which could enhance the structural diversity and complexity for these benzo-fused six- to eight-membered N-heterocycles would be genuinely attractive.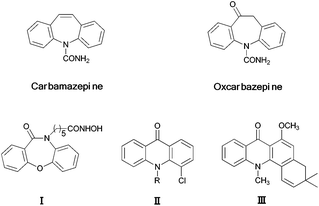 | ||
| Fig. 1 Examples of drugs, biologically active compounds and natural products containing the benzo-fused N-heterocycle motif. | ||
 | ||
| Scheme 1 The strategy for the synthesis of four kinds of six- to eight-membered, benzo-fused N-heterocycles. | ||
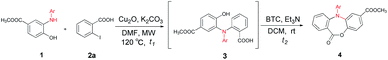
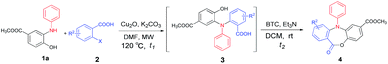
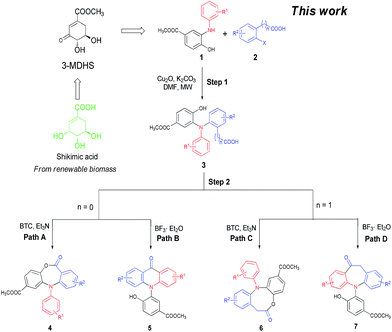
With the diminishing of fossil oil reserves and the increasing concerns about the environment and sustainability, the development of novel and efficient biomass-involved strategies to meet our needs for chemical and pharmaceutical products has drawn worldwide attention.10 In the last decade, chemo- and regio-selective synthesis of intermediates and fine chemicals from renewable biomass or biomass-derived substrates represents one of the most important innovations in organic chemistry.11 Our recent studies showed that the methyl 3-dehydroshikimate (3-MDHS), which was readily synthesized from (−)-shikimic acid could serve as a versatile and highly efficient reagent for various N-arylation processes, and could be used to generate a series of platform compounds, namely N-substituted 2-aminophenols (1)12 (Scheme 1). We envisioned that 1 could readily couple with 2-halogenated benzoic acids or 2-halogenated phenylacetic acids to afford the mutlifunctionalized triarylamines (3), which could serve as successive platform compounds with great potential for further transformations. Subsequent cyclization under different conditions would give rise to a range of N-arylated benzo-fused heterocycles (Scheme 1). As a part of our continuing efforts towards biomass conversion and the biomass-involved construction of bioactive molecules,13 we herein report a chemo- and regio-selective strategy for the facile synthesis of four kinds of novel six- to eight-membered, benzo-fused N-heterocycles from the renewable feedstock (−)-shikimic acid (Scheme 1).
Results and discussion
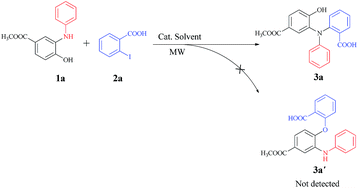
Based on the well-established protocol for the preparation of N-aryl 2-aminophenols (1) from the abundantly available (−)-shikimic acid,12 we chose the reaction of 1a and 2-iodobenzoic acid (2a) as the model reaction to screen conditions for access to triarylamine (3a) (Scheme 1, step 1) under microwave irradiation (Table 1). To our delight, the N-arylation reaction proceeded smoothly in the presence of Cu2O (20 mol%) in DMF with high chemo-selectivity providing the highly functionalized triarylamine (3a) in good yield (87%), whereas no O-arylated product (3a′) was detected (Table 1, entry 1). Other copper catalysts, such as Cu(OAc)2, CuSO4, Cu(NO3)2 were used, but could only afford 3a in moderate yields (Table 1, entries 2–4). Interestingly, a blue solution was formed in the work-up procedure when we diluted the filtrate of the reaction mixture with water (20% Cu2O was used as catalyst, Table 1, entry 1), which indicated that the more catalytically active Cu(I) species might be partially oxidized to the less active Cu(II) species during the reaction under air conditions. Hence, control experiments were carried out with Cu2O (20 mol%) used as the catalyst under N2 or O2 atmosphere. As shown in Table 1, an excellent yield (95%) of 3a was obtained when the N-arylation reaction was performed under N2 atmosphere (Table 1, entry 5), while only a moderate yield was obtained when the reaction was carried out under O2 atmosphere (Table 1, entry 6). When increasing the amount of catalyst to 1 equiv., no obvious improvement of the yield was observed (Table 1, entry 7). Unexpectedly, when 20 mol% 1,10-phenanthroline or 2,2′-bipyridine were used as the ligand, a sharp decrease of the yield was observed, which might be due to side reactions such as decarboxylation reaction being accelerated by the ligands. In comparison, the reaction was also carried out in an oil bath at 120 °C for 5 hours and a slightly lower yield was obtained (89%, Table 1, entry 10). It is worth mentioning that, although other inorganic bases, such as Na2CO3, Cs2CO3 were also effective for this copper catalyzed chemo-selective N-arylation reaction (Table 1, entries 11 and 12), we eventually chose K2CO3 as the base for economic considerations. Thus, the optimized reaction conditions for the construction of the triarylamine intermediate 3a (step 1) consist of 1a (1 mmol), 2 (0.25 g, 1 mmol), DMF (5 ml), Cu2O (20 mol%), and K2CO3 (3 mmol) under N2 atmosphere in microwave conditions.| Entry | Catalyst | Base | t1 (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.24 g, 1 mmol), 2a (0.25 g, 1 mmol), DMF (5 ml), catalyst (20 mol%), base (3 mmol) at 120 °C for indicated minutes under N2 atmosphere.b Isolated yields.c Reaction carried out in air.d Reaction carried out in O2 atmosphere.e 1 mmol Cu2O used as the catalyst.f 20% mmol 1,10-phenanthroline used as the ligand.g 20% mmol 2,2′-bipyridine used as the ligand.h Reaction carried out in an oil bath. | ||||
| 1c | Cu2O | K2CO3 | 8 | 87 |
| 2c | Cu(OAc)2 | K2CO3 | 10 | 56 |
| 3c | CuSO4 | K2CO3 | 10 | 60 |
| 4c | Cu(NO3)2 | K2CO3 | 10 | 53 |
| 5 | Cu2O | K2CO3 | 8 | 95 |
| 6d | Cu2O | K2CO3 | 8 | 72 |
| 7e | Cu2O | K2CO3 | 6 | 95 |
| 8f | Cu2O | K2CO3 | 6 | 52 |
| 9g | Cu2O | K2CO3 | 6 | 69 |
| 10h | Cu2O | K2CO3 | 300 | 89 |
| 11 | Cu2O | Na2CO3 | 8 | 87 |
| 12 | Cu2O | Cs2CO3 | 8 | 95 |
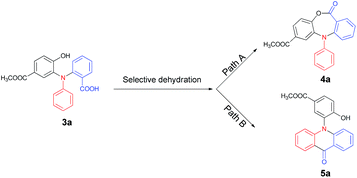
The thus obtained highly functionalized triarylamines 3 which contained multiple active groups, displayed a good opportunity for further chemo-selective manipulations. We deduced that the N-aryl dibenzo[b,e][1,4]oxazepin-11(5H)-ones (4) and the N-aryl acridones (5) could be obtained respectively via a dehydration process, one through intramolecular esterification (Scheme 1, Path A) and the other through intramolecular acylation (Scheme 1, Path B). Accordingly, 3a was chosen as the model substrate to screen the catalysts suitable for these transformations (Table 2, entries 1–7). Results showed that treatment of 3a with PTSA (10%) in toluene for 8 h gave rise to the esterification product 4a in 30% yield and the acylation product 5a in 55% yield (Table 2, entry 1). The same reaction promoted by SOCl2 (2 equiv.) in dichloromethane in an ice bath afforded 4a with a higher selectivity (76% for 4a, 10% for 5a, Table 2, entry 2). We have also found that 4a could be obtained in 62% yield in the presence of DCC/DMAP in THF at 65 °C for 12 h, whereas 5a was not detected in the reaction mixture (Table 2, entry 3). Satisfactorily, when bis(trichloromethyl)carbonate (BTC) was used as the catalyst, the esterification reaction was performed selectively and efficiently at room temperature, affording 4a in 96% yield with no 5a obtained (Table 2, entry 4). Attempts for selective construction of 5a were also made. We have found that, although the polyphosphoric acid (PPA) had previously proven to be an efficient catalyst for acylation reactions,14 it failed to convert 3a into 5a in the present conditions, presumably due to the side reaction cased by the hydroxyl group (Table 2, entry 5). Much to our surprise, when BF3·Et2O was used as the catalyst, the acylation reaction proceeded efficiently with high chemo- and regio-selectivity, affording 5a in 92% yield, whereas no 4a was detected (Table 2, entry 6). However, only a trace amount of 5a was obtained when 3a was treated with 4 equiv. AlCl3 in DCM for 12 h (Table 2, entry 7). Thus, the optimized reaction conditions for the selective formation of 4a and 5a include, treatment of 3a (1 mmol) with BTC (0.30 g, 1 mmol) in the presence of Et3N (4 mmol) in DCM gave 4a (Table 2, Path A), and treatment of 3a (1 mmol) with BF3·Et2O afforded 5a (Table 2, Path B).
| Entry | Catalyst (Path A) | Catalyst (Path B) | t2 (h) | Yielda (%) (4a) | Yielda (%) (5a) |
|---|---|---|---|---|---|
| a Isolated yield.b ND: not detected.c Reaction conditions: 3a (0.36 g, 1 mmol), PTSA (0.1 mmol), toluene (5 ml) reflux for indicated hours.d Reaction conditions: 3a (0.36 g, 1 mmol), DCC (0.41 g, 2 mmol), DMAP (12 mg, 0.1 mmol) and THF (5 ml).e Reaction conditions: 3a (0.36 g, 1 mmol), BTC (0.29 g, 1 mmol), Et3N (4 mmol) and DCM (5 ml).f Reaction conditions: 3a (0.36 g, 1 mmol) and PPA (5 ml) heated at 100 °C for indicated hours.g Reaction conditions: 3a (0.36 g, 1 mmol) in BF3·Et2O (5 ml) heated at 60 °C for 3 h. | |||||
| 1c | PTSA | — | 8 | 30 | 55 |
| 2 | SOCl2 | — | 5 | 76 | 10 |
| 3d | DCC/DMAP | — | 12 | 62 | NDb |
| 4e | BTC | — | 3 | 96 | NDb |
| 5f | — | PPA | 2 | Trace | Trace |
| 6g | — | BF3·Et2O | 3 | NDb | 92 |
| 7 | — | AlCl3 | 12 | NDb | Trace |
With the optimized reaction conditions in hand, we further investigated the substrate scope for the synthesis of N-aryl dibenzo[b,e][1,4]oxazepin-11(5H)-ones (4). As summarized in Table 3, a wide range of functional groups, such as Me, OMe, F, Cl, Br, NO2 and CF3 were compatible in this two step transformation, affording the corresponding products (4a–4p) in very good to excellent yields. In general, substrates possessing an electron-donating group tended to give better yields than those bearing an electron-withdrawing group. For example, 1b and 1c gave rise to 4b and 4c in excellent yields (Table 3, entries 2 and 3), whereas 1g and 1h afforded 4g and 4h in moderate yields (Table 3, entries 7 and 8). A steric effect was also observed. Substrates which were ortho-substituted, such as 1i and 1j afforded the corresponding products 4i and 4j in relatively lower yields (Table 3, entries 9 and 10). To our satisfaction, the N-naphthyl substituted substrate 1o as well as the N-biphenyl substituted substrate 1p were also compatible under the optimized reaction conditions and furnished the desired products 4o and 4p (Table 3, entries 15 and 16) in moderate and good yields.
| Entry | 1 | Ar | t1/t2 (min h−1) | Product | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (1 mmol), 2-iodobenzoic acid (0.25 g, 1.0 mmol), K2CO3 (3 mmol), DMF (5 ml) and Cu2O (20 mol%) at 120 °C for indicated minutes (t1) under microwave irradiation with the protection of N2 atmosphere (step 1); the isolated intermediate 3, BTC (0.30 g, 1 mmol) and Et3N (4 mmol) in DCM (5 ml) at room temperature for t2 hours (step 2).b Isolated yields based on 1. | |||||
| 1 | 1a | Ph | 8/3 | 4a | 91 |
| 2 | 1b | 4-MeC6H4 | 8/3 | 4b | 91 |
| 3 | 1c | 4-OMeC6H4 | 8/3 | 4c | 93 |
| 4 | 1d | 4-FC6H4 | 10/4 | 4d | 83 |
| 5 | 1e | 4-ClC6H4 | 10/3 | 4e | 85 |
| 6 | 1f | 4-BrC6H4 | 10/3 | 4f | 85 |
| 7 | 1g | 4-AcC6H4 | 12/4 | 4g | 78 |
| 8 | 1h | 4-NO2C6H4 | 12/4 | 4h | 80 |
| 9 | 1i | 2-MeC6H4 | 12/4 | 4i | 87 |
| 10 | 1j | 2,5-Cl2C6H3 | 15/5 | 4j | 65 |
| 11 | 1k | 3-MeC6H4 | 8/3 | 4k | 90 |
| 12 | 1l | 3-ClC6H4 | 10/3 | 4l | 83 |
| 13 | 1m | 3-NO2C6H4 | 12/4 | 4m | 82 |
| 14 | 1n | 3-CF3C6H4 | 13/4 | 4n | 75 |
| 15 | 1o | 1-Naphthyl | 17/5 | 4o | 68 |
| 16 | 1p | 4-(4-ClC6H4)C6H4 | 10/3 | 4p | 85 |
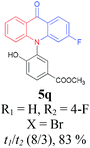
Inspired by these encouraging results, we next explored the scope of substrate 2 using various 2-halogenated benzoic acids under the optimized reaction conditions (Table 4). Results showed that 2-iodobenzoic acid (2a), 2-bromobenzoic acid (2b) as well as 2-chlorobenzoic acid (2c) could chemo-selectively couple with 1a to afford the corresponding triarylamine 3a, which could subsequently undergo the intramolecular esterification to furnish the desired product 4a in moderate to excellent yields (Table 4, entries 1–3). However, no desired product was detected when 2-fluorobenzoic acid (2d) was used as the reactant (Table 4, entry 4). Gratifyingly, 2-halogenated benzoic acids with an additional substituent, such as –F or –NO2 were also workable in this process. For example, the desired products 4q and 4r were obtained in 87% (entry 5) and 79% yield (entry 6) respectively, when 2-bromo-4-fluorobenzoic acid (2e) and 2-chloro-5-nitrobenzoic acids (2f) were used as the substrates under the optimized reaction conditions.
| Entry | 2 | R2 | t1/t2 (min h−1) | Product | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (1 mmol), 2-halogenated benzoic acids (1 mmol), K2CO3 (3 mmol) and DMF (5 ml) with Cu2O (20 mol%) as catalyst for step 1; the isolated intermediate 3, BTC (0.30 g, 1 mmol) and Et3N (4 mmol) in DCM (5 ml) for step 2.b Isolated yield.c ND: not detected. | |||||
| 1 | 2a, X = I | H | 8/3 | 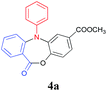 |
91 |
| 2 | 2b, X = Br | H | 10/3 | 85 | |
| 3 | 2c, X = Cl | H | 20/3 | 62 | |
| 4 | 2d, X = F | H | 30/— | NDc | |
| 5 | 2e, X = Br | 4-F | 8/4 | 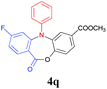 |
87 |
| 6 | 2f, X = Cl | 5-NO2 | 12/4 | 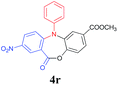 |
79 |
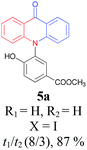
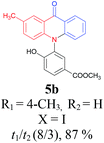
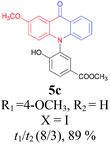
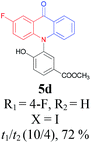
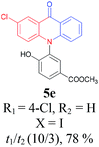
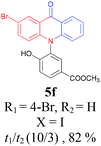
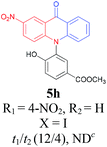
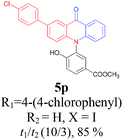
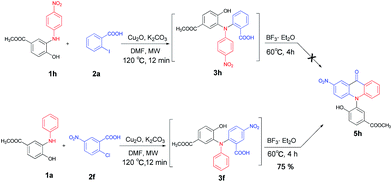
Under the aforementioned optimal reaction conditions for the synthesis of N-aryl acridones, a wide variety of N-arylated 2-aminophenols (1a–1f, 1h and 1p) and 2-halogen benzoic acids (2a and 2e) were subjected to the reaction conditions (Table 5). Intriguingly, the intramolecular acylation reaction of functionalized triarylamine intermediates 3 occurred selectively at A-ring, leading to the desired products 5 in moderate to good yields, whereas 5′ to which the acylation reaction should take place at B-ring was not detected. As expected, electron-donating groups or halogen atoms on A-ring were beneficial to the acylation reaction. Substrates bearing a Me, OMe, Cl and Br group (1b, 1c, 1e and 1f) performed smoothly in this two-step transformations and gave rise to the desired products 5b, 5c, 5e and 5f in very good to excellent yields. Even with a strong electron-withdrawing group, such as the fluorinated substrate 1d (R1 = 4-F), a 72% yield of the desired product 5d could still be obtained by reacting with 2-iodobenzoic acid. However, no acylation product 5h was detected in the case of 1h bearing the strong electron-withdrawing 4-nitro group (Scheme 2 and Table 5) Fortunately, we still managed to obtain 5h in a 75% yield when 1a (R1 = H) and 2f (X = Cl, R2 = 5-NO2) were used as the reactants (Scheme 2). This interesting result provided an alternative and effective approach towards the N-aryl acridones bearing electron-withdrawing group(s) in one of the aryl rings. It is worth noting that, biphenyl substrate 1p was also compatible and afforded the desired product 5p in 85% yield. Moreover, fluorine substituted 2-halogenated benzoic acids such as 2-bromo-4-fluorobenzoic acid (2e) could also be converted to N-arylacridone (5q) in good yield (83%).
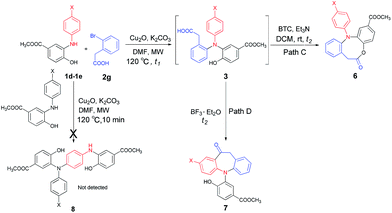
Finally, the substrate scope of this chemo- and regio-selective strategy based upon the biomass-derived N-aryl 2-aminophenols (1) was also examined using 2-bromophenylacetic acid (2g) as the reactant. Likewise, the coupling reaction between 1 and 2g readily afforded the key triarylamine intermediates 3 (Table 6), which would then undergo lactonization or acylation in the presence of BTC/Et3N or BF3·Et2O, respectively (Scheme 1, Path C and D), and selectively provide the benzo-fused, eight-membered N-aryl lactone products 6d and 6e or seven-membered N-aryl acylation products 7d and 7e in good yields (Table 6). It is noteworthy that, high chemo- and regio-selectivity was also observed in this two step transformation, as no self-condensation products (8) were detected in the reaction mixture. Notably, the acylation process (Path D) provided an efficient entry for the assembly of the novel N-aryl oxcarbazepine derivatives, which had not been reported elsewhere. In addition, the structure of all of the compounds reported in this paper were unambiguously confirmed by MS, 1H NMR, 13C NMR, HRMS and FT-IR analysis.
| Entry | X | t1/t2 (min h−1) | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), 2-bromophenylacetic acid (1.0 mmol), K2CO3 (3.0 mmol), DMF (5.0 ml) and Cu2O (20 mol%) reacted at 120 °C for t1 minutes (step 1); the isolated intermediate 3, DCM (5 ml) and BTC (0.30 g, 1 mmol) using Et3N (4 mmol) for step 2 (Path C) or the isolated intermediate 3 and BF3·Et2O (5.0 ml) reacted at 60 °C for t2 h for step 2 (Path D).b Isolated yields based on 1. | ||||
| 1 | F | 10/4 | 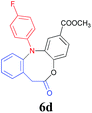 |
85 |
| 2 | Cl | 8/3 | 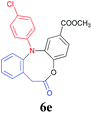 |
89 |
| 3 | F | 10/3 | 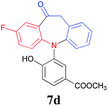 |
78 |
| 4 | Cl | 8/3 | 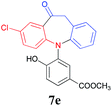 |
80 |
Conclusion
In conclusion, we have developed an efficient and practical method for the facile construction of six- to eight-membered, benzo-fused N-heterocycles from the biomass-derived N-aryl 2-aminophenols (1). A series of N-aryl-dibenzo[b,e][1,4]oxazepin-11(5H)-ones (4), N-aryl-acridones (5), N-aryl-7,12-dihydrodibenzo[b,e][1,4]oxazocin-6(H)-ones (6) as well as N-aryl-dibenzo[b,f]azepin-10(11H)-ones (7) were efficiently synthesized via an N-arylation reaction (step 1) and a controllable, chemo- and regio-selective intramolecular dehydration reaction (step 2). The utilization of biomass-derived platform compounds, the operational simplicity, the controllable selectivity, the structural novelty and diversity of compounds, are notable features of this protocol and conform to the elements of green chemistry. In addition, the six- to eight-membered, benzo-fused N-heterocycles with novel structural characteristics might be beneficial in drug development.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21272280, 81201716), Science & Technology Program of Guangdong Province and Guangzhou City (2011A081401002, 2014J4100224), Guangdong Natural Science Foundation (S2013010014278), and Guangdong Provincial Key Laboratory of Construction Foundation (2011A060901014). The authors are also thankful to Guangxi Wanshan Spice Co. Ltd. for giving high quality (−)-shikimic acid as a gift.Notes and references
- (a) J. Kricka and A. Ledwith, Chem. Rev., 1974, 74, 101 CrossRef; (b) O. Tabarrini, V. Cecchetti, A. Fravolini, G. Nocentini, A. Barzi, S. Sabatini, H. Miao and C. Sissi, J. Med. Chem., 1999, 42, 2136 CrossRef CAS PubMed.
- G. Bellucci, G. Berti, C. Chiappe, A. Lippi and F. Marioni, J. Med. Chem., 1987, 30, 768 CrossRef CAS.
- (a) M. Carril, R. SanMartin, F. Churruca, I. Tellitu and E. Dominguez, Org. Lett., 2005, 7, 4787 CrossRef CAS PubMed; (b) P. C. Fuenfschilling, W. Zaugg, U. Beutler, D. Kaufmann, O. Lohse, J. P. Mutz, U. Onken, J. L. Reber and D. Shenton, Org. Process Res. Dev., 2005, 9, 272 CrossRef CAS.
- M. Bigioni, M. Parlani, A. Giolitti, M. Fratelli, C. Valli, M. Terao and E. Garattini, ACS Med. Chem. Lett., 2010, 1, 411 CrossRef PubMed.
- V. V. S. R. Prasad, J. V. Rao, R. S. Giri, N. K. Sathish, S. M. S. Kumar and Y. C. Mayur, Chem.-Biol. Interact., 2008, 176, 212 CrossRef CAS PubMed.
- (a) P. Magiatis, S. Mitaku, A. L. Skaltsounis, F. Tillequin, A. Pierre and G. Atassi, Nat. Prod. Lett., 2000, 14, 183 CrossRef CAS; (b) A. Putic, L. Stecher, H. Prinz and K. Muller, Eur. J. Med. Chem., 2010, 45, 3299 CrossRef CAS PubMed.
- (a) Y. L. Liu, C. X. Chu, A. P. Huang, C. J. Zhan, Y. Ma and C. Ma, ACS Comb. Sci., 2011, 13, 547 CrossRef CAS PubMed; (b) S. M. Lu and H. Alper, J. Am. Chem. Soc., 2005, 127, 14776 CrossRef CAS PubMed; (c) Q. A. Yang, H. Cao, A. Robertson and H. Alper, J. Org. Chem., 2010, 75, 6297 CrossRef CAS PubMed.
- (a) P. C. Huang, K. Parthasarathy and C. H. Cheng, Chem.–Eur. J., 2013, 19, 460 CrossRef CAS PubMed; (b) W. Zhou, Y. Liu, Y. Q. Yang and G. J. Deng, Chem. Commun., 2012, 48, 10678 RSC.
- (a) J. Kim and B. M. Stoltz, Tetrahedron Lett., 2012, 53, 4994 CrossRef CAS PubMed; (b) D. G. Pintori and M. F. Greaney, Org. Lett., 2010, 12, 168 CrossRef CAS PubMed.
- (a) F. Cherubini, Energy Convers. Manage., 2010, 51, 1412 CrossRef CAS PubMed; (b) A. Uihlein and L. Schebek, Biomass Bioenergy, 2009, 33, 793 CrossRef CAS PubMed; (c) Y. G. Zheng, X. L. Chen and Y. C. Shen, Chem. Rev., 2008, 108, 5253 CrossRef CAS PubMed; (d) B. Kamm, Angew. Chem., Int. Ed., 2007, 46, 5056 CrossRef CAS PubMed; (e) Á. Szabolcs, M. Molnár, G. Dibó and L. T. Mika, Green Chem., 2013, 15, 439 RSC; (f) R. J. Putten, J. C. Waal, E. Jong, C. B. Rasrendra, H. J. Heeres and J. G. Vries, Chem. Rev., 2013, 113, 1499 CrossRef PubMed; (g) R. Karinen, K. Vilonen and M. Niemel, ChemSusChem, 2011, 4, 1002 CrossRef CAS PubMed; (h) A. Behr and L. Johnen, ChemSusChem, 2009, 2, 1072 CrossRef CAS PubMed; (i) J. J. Wang, X. H. Liu, B. C. Hu, G. Z. Lu and Y. Q. Wang, RSC Adv., 2014, 4, 31101 RSC; (j) A. W. Bhutto, K. Qureshi, K. Harijan, G. Zahedi and A. Bahadori, RSC Adv., 2014, 4, 3392 RSC; (k) X. R. Liu, X. C. Wang, S. X. Yao, Y. J. Jiang, J. Guan and X. D. Mu, RSC Adv., 2014, 4, 49501 RSC; (l) F. Liu, F. Boissou, A. Vignault, L. Lemée, S. Marinkovic, B. Estrine, K. D. O. Vigier and F. Jérôme, RSC Adv., 2014, 4, 28836 RSC.
- (a) S. Kambourakis and J. W. Frost, J. Org. Chem., 2000, 65, 6904 CrossRef CAS PubMed; (b) W. S. Li, D. M. Xie and J. W. Frost, J. Am. Chem. Soc., 2005, 127, 2874 CrossRef CAS PubMed; (c) L. D. Nie, X. X. Shi, K. H. Ko and W. D. Lu, J. Org. Chem., 2009, 74, 3970 CrossRef CAS PubMed; (d) N. Q. Ran, D. R. Knop, K. M. Draths and J. W. Frost, J. Am. Chem. Soc., 2001, 123, 10927 CrossRef CAS PubMed; (e) Y. J. Pagaán-Torres, T. F. Wang, J. M. R. Gallo, B. H. Shanks and J. A. Dumesic, ACS Catal., 2012, 2, 930 CrossRef; (f) L. Deng, J. Li, D. M. Lai, Y. Fu and Q. X. Guo, Angew. Chem., Int. Ed., 2009, 48, 6529 CrossRef CAS PubMed; (g) C. A. Hansen and J. W. Frost, J. Am. Chem. Soc., 2002, 124, 5926 CrossRef CAS PubMed; (h) J. M. Gibson, P. S. Thomas, J. D. Thomas, J. L. Barker, S. S. Chandran, M. K. Harrup, K. M. Draths and J. W. Frost, Angew. Chem., Int. Ed., 2001, 40, 1945 CrossRef CAS; (i) E. Arceo, J. A. Ellman and R. G. Bergman, ChemSusChem, 2010, 3, 811 CrossRef CAS PubMed.
- W. Wu, Y. Zou, Y. Chen, J. Li, Z. L. Lv, W. Wei, T. K. Huang and X. K. Liu, Green Chem., 2012, 14, 363 RSC.
- (a) Y. Chen, Y. Zou, H. Y. Sun, X. K. Liu, C. F. Xiao, J. Sun, S. J. He and J. Li, Synthesis, 2011, 43, 217 Search PubMed; (b) E. S. Zhang, T. L. Xu, W. Wei, T. K. Huang, M. Yuan, W. Zeng and Y. Zou, Synthesis, 2014, 46, 1167 CrossRef PubMed; (c) Y. Zou, Q. Huang, T. K. Huang, Q. C. Ni, E. S. Zhang, T. L. Xu, M. Yuan and J. Li, Org. Biomol. Chem., 2013, 11, 6967 RSC; (d) Y. Zou, E. S. Zhang, T. L. Xu, W. Wu, Y. Chen, M. Yuan, W. Wei and X. J. Zhang, RSC Adv., 2013, 3, 6545 RSC; (e) E. S. Zhang, T. L. Xu, D. J. Wang, T. K. Huang, M. Yuan, J. Li and Y. Zou, RSC Adv., 2014, 4, 10022 RSC; (f) E. S. Zhang, X. J. Zhang, Y. C. Cai, D. J. Wang, T. L. Xu, J. Li, M. Yan and Y. Zou, RSC Adv., 2014, 4, 39020 RSC.
- (a) P. Huszthy, Z. Kontos, B. Vermes and A. Pinter, Tetrahedron, 2001, 57, 4967 CrossRef CAS; (b) S. H. Watterson, P. Chen, Y. Zhao, H. H. Gu, T. G. M. Dhar, Z. Xiao, S. K. Ballentine, Z. Shen, C. A. Fleener, K. A. Rouleau, M. Obermeier, Z. Yang, K. W. McIntyre, D. J. Shuster, M. Witmer, D. Dambach, S. Chao, A. Mathur, B. C. Chen, J. C. Barrish, J. A. Robl, R. Townsend and E. J. Iwanowicz, J. Med. Chem., 2007, 50, 3730 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c4ra12479k |
| This journal is © The Royal Society of Chemistry 2015 |