Organoiodine(III) mediated intramolecular oxidative cyclization of 1-(3-arylisoquinolin-1-yl)-2-(arylmethylene)hydrazines to 5-aryl-3-(aryl)-[1,2,4]triazolo[3,4-a] isoquinolines†
Pitchai Manivela,
Kamalakannan Prabakarana,
Upasana Banerjeea,
Fazlur-Rahman Nawaz Khan*ab,
Euh Duck Jeong*b and
Eun Hyuk Chungb
aOrganic and Medicinal Chemistry Research Laboratory, Organic Chemistry Division, School of Advanced Sciences, VIT-University, Vellore, 632 014, Tamil Nadu, India. E-mail: nawaz_f@yahoo.co.in; fnkn@kbsi.re.kr
bKorea Basic Science Institute, Busan Center, Busan 618 230, South Korea. E-mail: edjeong@kbsi.re.kr
First published on 2nd December 2014
Abstract
A series of 5-aryl-3-(aryl)-[1,2,4]triazolo[3,4-a]isoquinolines, 4 were obtained by oxidative cyclization of 1-(3-arylisoquinolin-1-yl)-2-(arylmethylene)hydrazines, 3, in the presence of a hypervalent iodine oxidant (iodobenzene diacetate, IDB) and dichloromethane at ambient temperature. This methodology involves a proficient metal-free intramolecular C–N bond formation, facilitated by a hypervalent iodine reagent.
Introduction
Isoquinoline, an imperative heterocyclic template of an assortment of natural products and pharmaceuticals, possesses intriguing biological activity.1,2 Further, 1,2,4-triazoles find their application in the fields of biological and pharmacological activities,3–5 including antifungal,6 bactericidal,6,7 anxiolytic,8,9 anticonvulsant,10 or herbicidal11 and antidepressant activities.12 It is fascinating that hydrazine and its analogs promptly undergo annulations to the 1,2,4-triazole ring,13 a guaranteed biologically active compound.14,15 Likewise, the fused heterocyclic 1,2,4-triazoles find their essentialness as CNS depressant,16 antiallergy,17 antimicrobial18 and anti-inflammatory19 drugs. In this way, it was envisaged that chemical entities with both the isoquinoline and fused or bridged 1,2,4-triazole might result in compounds with interesting biological activity. In this perspective and with our longstanding interest in the synthesis and diversification of heterocycles, particularly in quinoline and isoquinoline chemistry,20–22 thus we report a convenient, practical and efficient hypervalent iodine mediated synthesis of bridgehead triazoles, for instance, 5-aryl-3-(aryl)-[1,2,4]triazolo[3,4-a]isoquinolines (Scheme 1).As of late, the triazolopyridines, triazoloquinolines and so on has been reported by oxidative cyclization from their respective hydrazones.23–35 Few of the strategies included the utilization of dangerous reagents like lead tetracetate, phosphorus oxychloride or a moisture sensitive ferric chloride, microwave irradiation in acetic anhydride or nitrobenzene which required reflux conditions.36–38 Likewise, few others included laborious procedures, larger amount of oxidants thereby causing environmental issues. To conquer these, alternate methods and reagents were developed.39,40 so that reactions are completed under the mild reaction conditions and acted as an option to the reported customary strategies. Amongst the organohypervalent iodine reagents (Fig. 1),41–46 the iodobenzene diacetate (IBD) have risen as a low toxic, readily accessible, and simplicity to handle reagent for the valuable transformations.47–60
Results and discussion
The synthetic pathway of 5-aryl-3-(aryl)-[1,2,4]triazolo[3,4-a]isoquinolines, 4 is depicted in Scheme 1. The imperative cyclization precursor hydrazones were promptly acquired by the condensation of 1-(3-arylisoquinolin-1-yl) hydrazine, 1 with distinctive aromatic and heteroaromatic aldehydes, 2 in isopropanol under reflux condition with a trace of glacial acetic acid. The 1-(3-arylisoquinolin-1-yl)-2-(arylmethylene)hydrazines, 3 formed were filtered, dried and utilized for further investigation on cyclization.At the outset, the reaction was done utilizing a mixture of 1-(3-phenylisoquinolin-1-yl)-2-(thiophen-2-ylmethylene) hydrazine, 3a (1 mmol) without the iodine reagent and solvent under heating condition at 100 °C, however, the reaction did not continue to give the sought 5-phenyl-3-(thiophen-2-yl)-[1,2,4]triazolo[3,4-a]isoquinoline, 4a (Scheme 1, Table 1, entry 1), whereas 17–30% of 4a is formed in the presence of iodine reagents in acetonitrile solvent (Table 1, entries 2–4). The results demonstrated that both the solvent and iodine reagents are essential for the reaction. Further, we shifted the solvents from CH3CN, toluene and CH2Cl2 (Scheme 1, Table 1, entries 5–10). Among the investigated conditions, in the presence of IBD, CH2Cl2, the reaction offered the desired product, 4a with a yield of 87% (Scheme 1, Table 1 and entry 8). Likewise, the IBTF and HTIB proceeded to offer the desired product in low yield of 20–46% after delayed reaction time (Scheme 1, Table 1, entries 5–7, 9, 10). Energized by these results, further improvement was carried out by altering iodine reagent loading under CH2Cl2 solvent reflux conditions. Amongst the different catalysts loading, good yields of 92% were obtained utilizing 1.1 equiv. of IBD/CH2Cl2 system (Table 2, entry 13) and other trials gave moderate yields (Table 2, entries 1–12). Treatment of 1-(3-arylisoquinolin-1-yl)-2-(arylmethylene)hydrazine, 3 with iodobenzenediacetate (IBD) in dichloromethane for 1 h at room temperature brought out the cyclization in the establishment of a solitary product.
| Entry | Iodine(III) reagent (equiv.) | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: (i) 1-(3-phenylisoquinolin-1-yl)-2-(thiophen-2-ylmethylene) hydrazine, 3a (1 mmol, 1.0 equiv.), iodine reagent (1.0 equiv.) in solvent (10 ml) at room temperature for 1 h.b Isolated yields. | |||
| 1 | Nil | Nil | No reaction |
| 2 | IBD | CH3CN | 30 |
| 3 | IBTF | CH3CN | 21 |
| 4 | HTIB | CH3CN | 17 |
| 5 | IBD | Toluene | 25 |
| 6 | IBTF | Toluene | 20 |
| 7 | HTIB | Toluene | 28 |
| 8 | IBD | CH2Cl2 | 87 |
| 9 | IBTF | CH2Cl2 | 46 |
| 10 | HTIB | CH2Cl2 | 32 |
| Entry | Iodobenzene diacetate, IBD (equiv.) | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: (i) 1-(3-phenylisoquinolin-1-yl)-2-(thiophen-2-ylmethylene) hydrazine, 3a (1 mmol, 1.0 equiv.), iodine reagent in solvent (10 ml) at room temperature for 1 h.b Isolated yields. | |||
| 1 | 1 | Nil | 12 |
| 2 | 1 | DMF | 30 |
| 3 | 1 | Dioxane | 30 |
| 4 | 1 | THF | 30 |
| 5 | 1 | Toluene | 25 |
| 6 | 1 | CH3CN | 30 |
| 7 | 1 | CH2Cl2 | 87 |
| 8 | 0.1 | CH2Cl2 | 30 |
| 9 | 0.3 | CH2Cl2 | 55 |
| 10 | 0.5 | CH2Cl2 | 70 |
| 11 | 0.7 | CH2Cl2 | 74 |
| 12 | 0.9 | CH2Cl2 | 80 |
| 13 | 1.1 | CH2Cl2 | 92 |
The oxidative cyclization of hydrazones brought about the formation of 5-aryl-3-(aryl/heteroaryl)-[1,2,4]triazolo[3,4-a]isoquinolines, 4 (see Table 3). The oxidative transformation is clean and proficient. The 1-(3-arylisoquinolin-1-yl)-2-(arylmethylene)hydrazine, 3 of aromatic and heteroaromatic aldehydes, 2 with both electron-withdrawing and electron-donating substituent were oxidized to give the corresponding 5-aryl-3-(aryl)-[1,2,4]triazolo[3,4-a]isoquinolines, 4 in high yields (Table 3). The aliphatic aldehyde hydrazones did not proceed well to give the desired triazoles due to their immediate cleavage and oxidation of aldehyde functionality. The experimental procedure is exceptionally simple. The high yield transformation did not form any undesirable by-products. Furthermore, the products were procured with a higher degree of purity which obliged no further purification. The critical advantages of this strategy are operational straightforwardness, short reaction time, pure products, economical, and nontoxicity of the reagent and remarkable yields. The structures of every 5-aryl-3-(aryl)-[1,2,4]triazolo[3,4-a]isoquinolines, 4a–k were confirmed by their spectral data.
| a Reaction conditions: (i) 1-(3-arylisoquinolin-1-yl)-2-(arylmethylene) hydrazine, 3 (1 mmol, 1.0 equiv.), iodine reagent, IBD (1.1 equiv.) in dichloromethane (10 ml) at room temperature for 1 h. |
|---|
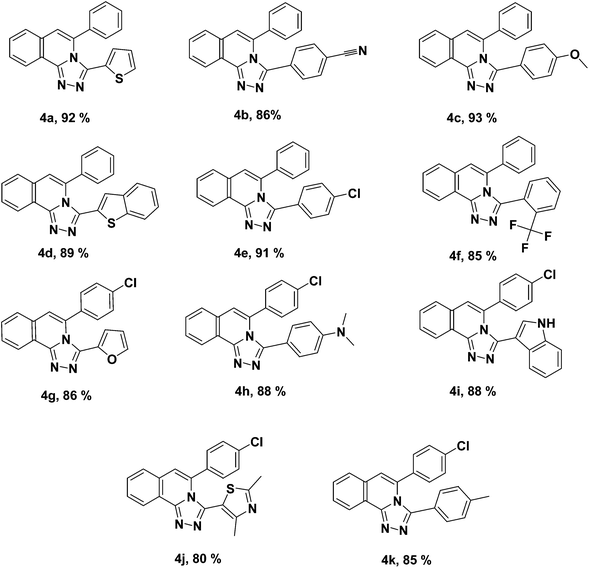 |
A conceivable mechanism is delineated in Scheme 2. The principal step includes the electrophilic attack of IBD on 1-(3-arylisoquinolin-1-yl)-2-(arylmethylene) hydrazine, 3 to form an organoiodine(III) intermediate, A. Consequently, A creates an alternate intermediate nitrile imide, B alongside ejection of molecules of iodobenzene and acetic acid. The nitrile amide, B experiences cyclization to give the 5-aryl-3-(aryl)-[1,2,4]triazolo [3,4-a]isoquinolines, 4.
Conclusions
The iodine(III)-mediated oxidative cyclization of 1-(3-aryllisoquinolin-1-yl)-2-(arylmethylene) hydrazine, 3 to 5-aryl-3-(aryl)-[1,2,4]triazolo [3,4-a]isoquinolines, 4 is significant as the strategy is eco-friendly, included gentle conditions, and there is a plausibility of utilizing this methodology for the synthesis of a wide variety of heterocyclic compounds of potential biological interest.Experimental
Typically, the condensation of 1-(3-phenylisoquinolin-1-yl) hydrazine, 1a (1 mmol), 2-thiophene-2-carboxaldehyde, 2a (1 mmol) in isopropanol (10 ml) under reflux condition with a trace of glacial acetic acid gave 1-(3-phenylisoquinolin-1-yl)-2-(thiophen-2-ylmethylene) hydrazine, 3a, which was filtered, dried and utilized for further investigation on cyclization.To a stirred solution of 1-(3-phenylisoquinolin-1-yl)-2-(thiophen-2-ylmethylene) hydrazine, 3a (1 mmol) in DCM (10 ml) at room temperature, IBD (1.1 mmol) was added in portions during 5 min. The resulting mixture was agitated for 1 h at room temperature. The solvent was evaporated and the residual mass containing product and iodobenzene triturated with petroleum ether to give the solid product, which was recrystallized from methanol to yield pure 5-phenyl-3-(thiophen-2-yl)-[1,2,4]triazolo[3,4-a]isoquinoline, 4a.
5-Phenyl-3-(thiophen-2-yl)-[1,2,4]triazolo[3,4-a]isoquinoline, (4a)
Brown solid, mp 195.1 °C, IR (KBr-ν cm−1): 3061, 1724, 1636, 1522, 1451, 1374, 1294, 1224, 1150, 844, 777, 762, 705, 538, 491. 1H NMR (400 MHz, DMSO-D6, 25 °C) δ ppm: 8.66 (d, J = 5.04 Hz, 1H), 7.99 (d, J = 7.12 Hz, 1H), 7.79 (m, 2H), 7.51 (d, J = 5.08 Hz, 1H), 7.34–7.15 (m, 6H), 6.66 (m, 1H), 6.50 (d, J = 3.60 Hz, 1H); 13CNMR (100 MHz DMSO-D6) δ ppm: 149.93, 143.71, 135.21, 133.32, 131.43, 130.83, 130.54, 129.49, 129.32, 128.97, 128.89, 128.17, 128.07, 127.84, 127.24, 123.38, 120.69, 117.36. Calcd for C20H13N3S, HRMS (EI) 327.0830; found, 327.1192 (M+).Acknowledgements
The authors wish to express their appreciation to the VIT University Vellore for research fund and facilities. This work was upheld by the Grant no. R0001026 from the Ministry of Trade, Industry & Energy and Busan Metropolitan City, Korea.Notes and references
- M. Croisy-Delcey, A. Croisy, D. Carrez, C. Huel, A. Chiaroni, P. Ducrot, E. Bisagni, L. Jin and G. Leclercq, Bioorg. Med. Chem., 2000, 8, 2629–2641 CrossRef CAS.
- K. W. Bentley, The Isoquinoline Alkaloids, Harwood Academic Publishers, Australia, 1998, vol. 1 Search PubMed.
- R. Sarges, H. R. Howord, R. G. Browne, L. A. Label, P. A. Seymour and B. K. Koe, J. Med. Chem., 1990, 33, 2240–2254 CrossRef CAS.
- H. L. Yale and J. J. Piala, J. Med. Chem., 1966, 9, 42–46 CrossRef CAS.
- N. Demirbas, S. A. Karaogly, A. Demirbas and K. Sancak, Eur. J. Med. Chem., 2004, 39, 793–804 CrossRef CAS PubMed.
- S. A. El-Hawash, N. S. Habib and N. H. Fanaki, Pharmazie, 1999, 54, 808–813 CAS.
- D. J. Brown and Y. Iwai, Aust. J. Chem., 1979, 32, 2727–2733 CrossRef CAS.
- G. Tarzia, E. Ocelli, E. Toja, D. Barone, N. Corsico, L. Gallico and F. Luzzani, J. Med. Chem., 1988, 31, 1115–1123 CrossRef CAS.
- R. I. Trust and J. D. Albright, U.S. Pat., 4 242 515, 1980.
- G. Tarzia, E. Ocelli and D. Barone, Il Farmaco, 1989, 44, 3–16 CAS.
- (a) R. Peignier, A. Chêne, R. Cantregril and J. Mortier, Eur. Pat., 41718 Chem. Abstr., 1991, 115, 208000; (b) R. Cantegril, A. Chêne, J. Mortier and R. Peignier, Eur. Pat., 483027 Chem. Abstr., 1992, 117, 131214.
- C. Ainsworth, N. R. Easton, M. Livezey, D. E. Morrison and W. R. Gibson, J. Med. Chem., 1962, 5, 383–389 CrossRef CAS.
- A. G. Mikhailovskii, V. S. Shiklyaev and E. V. Feshina, Chem. Heterocycl. Compd., 1998, 34, 211–215 CrossRef CAS.
- Yu. P. Kitaev and B. I. Buzykin, in Hydrazones, Nauka, Moscow, 1974 Search PubMed.
- E. M. Gizatullina and V. G. Kartsev, Khim. Geterotsikl. Soedin., 1993, 12, 1587 CAS.
- A. A. Deshmukh, M. K. Mody, T. Ramalingam and P. B. Sattur, Indian J Chem. B Org., 1984, 23, 793–795 Search PubMed.
- B. Loev, J. H. Musser, R. E. Brown, H. Jones, R. Kahen, F. C. Huang, A. Khandwala, P. Sonnio-Goldman and M. J. Leibowitz, J. Med. Chem., 1985, 28, 363–366 CrossRef CAS.
- S. P. Hiremath, A. Ullagaddi, K. Shivaramayya and M. G. Purohit, Indian J. Heterocycl. Chem., 1999, 3, 145–148 Search PubMed.
- R. Gupta, A. K. Gupta, S. Paul and P. L. Kachroo, Indian J. Chem., 1998, 37B, 1211–1213 CAS.
- (a) K. Prabakaran, F. Nawaz Khan and J. S. Jin, Tetrahedron Lett., 2011, 52, 2566–2570 CrossRef CAS PubMed; (b) K. Prabakaran, P. Manivel and F. Nawaz Khan, Tetrahedron Lett., 2010, 51, 4340–4343 CrossRef CAS PubMed.
- (a) P. Manivel, K. Prabakaran, V. Krishnakumar, F. Nawaz Khan and T. Maiyalagan, Ind. Eng. Chem. Res., 2014, 53, 7866–7870 CrossRef CAS; (b) P. Manivel, S. M. Roopan, R. S. Kumar and F. N. Khan, J. Chil. Chem. Soc., 2009, 54, 183–185 CAS.
- (a) K. Prabakaran, F. R. N. Khan, J. S. Jin, E. D. Jeong and P. Manivel, Chem. Pap., 2011, 65, 883–889 CrossRef CAS PubMed; (b) F. N. Khan, P. Manivel, K. Prabakaran, V. Hathwar and S. W. Ng, Acta Crystallogr., Sect. B: Struct. Sci., 2009, E66, o488 Search PubMed.
- R. Aggarwal and R. Kumar, Synth. Commun., 2009, 39, 2169–2177 CrossRef CAS.
- V. N. Telvekar and K. A. Sasane, Synlett, 2010, 2778–2779 CrossRef CAS PubMed.
- L. Emmanuvel, T. M. A. Shaikh and A. Sudalai, Org. Lett., 2005, 7, 5071–5074 CrossRef CAS PubMed.
- C. Tang, Z. Li and Q. Wang, Beilstein J. Org. Chem., 2013, 9, 2629–2634 CrossRef PubMed.
- S. Liu, J. Sawicki and T. G. Driver, Org. Lett., 2012, 14, 3744–3747 CrossRef CAS PubMed.
- J. Y. Roberge, G. Yu, A. Mikkilineni, X. Wu, Y. Zhu, R. M. Lawrence and W. R. Ewing, ARKIVOC, 2007, 132–147 CrossRef CAS.
- O. R. Thiel, M. M. Achmatowicz, A. Reichelt and R. D. Larsen, Angew. Chem., Int. Ed., 2010, 49, 8395–8398 CrossRef CAS PubMed.
- GmbH, Boehringer, Soehne, Br. Patent 1130909, Chem. Abstr., 1969, 70, 20090i.
- S. Naqui and K. R. Srinivasan, J. Sci. Ind. Res., 1962, 21B, 456–457 CAS.
- M. S. Gibson, Tetrahedron, 1963, 19, 1587–1589 CrossRef CAS.
- J. D. Bower and F. P. Doyle, J. Chem. Soc., 1957, 727–732 RSC.
- M. Kidwai, Y. Goel and R. Kumar, Indian J. Chem., 1998, 37B, 174–179 CAS.
- S. Naqui and K. R. Srinivasan, Indian J. Chem., 1965, 3B, 162–164 Search PubMed.
- B. Abarca, R. Adam, S. Alom, R. Ballesteros and S. López-Molina, ARKIVOC, 2014, 175–186 CAS.
- A. G. Arvanitis, P. J. Gilligan, J. P. Beck and B. Rajagopal, U S Pat., US 6,245,769 B1, 2001.
- A. S. Shawali, ARKIVOC, 2010, 33–97 CAS.
- P. Bourgeois, R. Cantegril, A. Chene, J. Gelin, J. Mortier and J. Moyroud, Synth. Commun., 1993, 23, 3195–3199 CrossRef CAS.
- S. Crljenak, I. Tabakovic, D. Jeremic and I. Gaon, Acta Chem. Scand., 1983, B 37, 527–535 CrossRef PubMed.
- R. M. Moriarty, J. Org. Chem., 2005, 70, 2893–2904 CrossRef CAS PubMed.
- G. F. Koser, Adv. Heterocycl. Chem., 2004, 86, 225–292 CrossRef CAS.
- T. Wirth, in Topics in Current Chemistry, ed. T. Wirth, Springer, Berlin, 2003, vol. 224 Search PubMed.
- V. V. Zhdankin and P. J. Stang, Chem. Rev., 2002, 102, 2523–2584 CrossRef CAS PubMed.
- (a) M. Ochiai, in Chemistry of Hypervalent Compounds, ed. K. Akibia, VCH, New York, 1999, ch. 13, pp. 359–387 Search PubMed; (b) A. Varvoglis, Hypervalent Iodine in Organic Synthesis, Academic, New York, 1997 Search PubMed.
- Y. Kita, H. Tohma and T. Yakura, Trends Org. Chem., 1992, 3, 113–128 CAS.
- R. M. Moriarty, C. J. Chany and J. W. Kosmeder, in Encyclopedia of Reagents in Organic Synthesis, ed. L. A. Paquette, John Wiley & Sons, Chichester, 1995, vol. 2, pp. 1479–1484 Search PubMed.
- O. Prakash and S. P. Singh, Aldrichimica Acta, 1994, 27, 15–22 CAS.
- A. Varvoglis, Chem. Soc. Rev., 1981, 10, 377–407 RSC.
- G. F. Koser, Aldrichimica Acta, 2001, 34, 89–102 CAS.
- O. Prakash, N. Saini and P. K. Sharma, Heterocycles, 1994, 38, 409–431 CrossRef CAS PubMed.
- R. M. Moriarty, R. K. Vaid and G. F. Koser, Synlett, 1990, 365–383 CrossRef CAS PubMed.
- O. Prakash, V. Sharma, V. Bhardwaj, R. Pundeer and H. Kaur, Tetrahedron Lett., 2004, 45, 9065–9067 CrossRef CAS PubMed.
- O. Prakash, R. Pundeer and H. Kaur, Synthesis, 2003, 2768–2770 CrossRef CAS.
- O. Prakash, V. Sharma and M. P. Tanwar, Can. J. Chem., 1999, 77, 1191–1195 CrossRef CAS.
- O. Prakash, H. Kaur, H. Batra, N. Rani and S. P. Singh, J. Org. Chem., 2001, 66, 2019–2023 CrossRef CAS PubMed.
- O. Prakash, A. Batra, V. Sharma, R. K. Saini and R. S. Verma, J. Indian Chem. Soc., 2003, 80, 1031–1033 CAS.
- O. Prakash, P. K. Sharma and N. Saini, Synlett, 1994, 221–227 CrossRef CAS PubMed.
- A. Sadana, Y. Mirza, K. R. Aneja and O. Prakash, Eur. J. Med. Chem., 2003, 38, 533–536 CrossRef CAS.
- O. Prakash, V. Bhardwaj, R. Kumar, P. Tyagi and K. R. Aneja, Eur. J. Med. Chem., 2004, 39, 1073–1077 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra12381f |
| This journal is © The Royal Society of Chemistry 2015 |



