ZnO/Ag micro/nanospheres with enhanced photocatalytic and antibacterial properties synthesized by a novel continuous synthesis method
Abstract
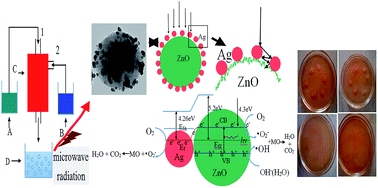
A series of ZnO/Ag micro/nanospheres (MNSs) with different Ag contents have been successfully synthesized via a self-design concentric impinging stream microreactor combined with microwave technique. Various characterization results showed that Ag nanoparticles with an average diameter of about 15 nm were successfully deposited upon ZnO microspheres with sizes ranging from 300–700 nm which are composed of ZnO nanoparticles with diameters of less than 10 nm. The photocatalytic performance of a series of ZnO/Ag MNSs was evaluated against methyl orange, and the antibacterial properties were tested against Escherichia coli. All of the results showed that the photocatalytic and antibacterial activities of ZnO/Ag MNSs loaded with different Ag contents are superior to that of the pure ZnO. The optimal loading Ag content is approximately 7.5 atom% as the MO is almost completely degraded after irradiating for 30 min and the MIC value is 100 μg mL−1. The possible mechanisms of the enhanced photocatalytic and antibacterial properties of ZnO/Ag MNSs were proposed. This fabrication method has the inherent advantages of simplicity, continuous production and low cost, so it is more appropriate for a large-scale continuous production with higher yields of ZnO/Ag MNSs in industry.
- This article is part of the themed collection: Chemistry for Medicine: Special Collection for RSC Advances

 Please wait while we load your content...
Please wait while we load your content...