Multi-functional DNA-based synthesis of SWNTs@(TiO2/Ag/Au) nanocomposites for enhanced light-harvesting and charge collection in DSSCs†
Abstract
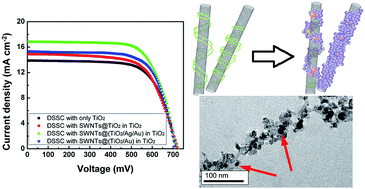
The performances of photovoltaic devices can be improved by increasing light-harvesting and charge collection respectively. The design and synthesis of nanocomposites with the ability of enhancing the generation and collection of the photo-generated electrons provide a significant way to improve the power conversion efficiency (PCE). Herein, SWNTs@(TiO2/Ag/Au) nanocomposites were synthesized and successfully integrated into photoanode films of dye-sensitized solar cells (DSSCs) to improve the power conversion efficiency. The synthesis processes were based on multi-functional DNA. DNA not only works as a dispersing agent preventing SWNTs bundling but also as a sacrificial mold assembling TiO2, Ag and Au nanoparticles on the surface of the SWNTs. The synthesized SWNTs@(TiO2/Ag/Au) nanocomposites enhance the charge collection and light-harvesting of DSSCs simultaneously. With 2.45 wt% SWNTs@(TiO2/Ag/Au) nanocomposites incorporating into the photoanode films, the PCE of the DSSCs increases from 6.8% to 8.3%.

 Please wait while we load your content...
Please wait while we load your content...