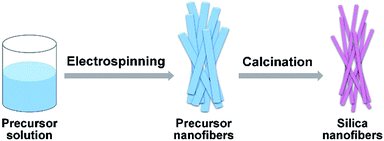
Silica nanofibrous membranes with ultra-softness and enhanced tensile strength for thermal insulation†
Yinsong Si‡
ab,
Xue Mao‡bc,
Hongxia Zhenga,
Jianyong Yub and
Bin Ding*ab
aKey Laboratory of Textile Science & Technology, Ministry of Education, College of Textiles, Donghua University, Shanghai 201620, China. E-mail: binding@dhu.edu.cn; Fax: +86-21-62378202; Tel: +86-21-62378202
bNanomaterials Research Center, Modern Textile Institute, Donghua University, Shanghai 200051, China
cState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, China
First published on 16th December 2014
Abstract
Novel silica nanofibrous (SNF) membranes with ultra-softness and enhanced tensile strength were prepared for the first time via an electrospinning technique with a sol–gel solution containing NaCl. By employing NaCl incorporation, the bonding structure was formed between silica nanofibers, which significantly enhanced the tensile strength of the SNF membranes from 3.2 to 5.5 MPa. Meanwhile, the morphology and mechanical properties of the SNF membranes can be finely controlled by tuning the calcination temperature and varying the NaCl content in the precursor solution. Additionally, the proposed mechanism of the softness of the SNF membranes was discussed by in situ SEM analysis during the bending and recovery process. Furthermore, the as-prepared SNF membranes with ultra-softness of 40 mN and relative high tensile strength of 5.5 MPa exhibit an ultra-low thermal conductivity of 0.0058 W m−1 K−1, even lower than air, which suggested them to be promising candidates for bunker clothing. This novel method also provides a new insight into the design and development of other soft ceramic nanofibrous membranes with high tensile strength for various applications.
Introduction
World energy consumption will rapid rise in the next three decades, driven by growth in developing countries such as China and India.1–3 The energy consumption is distributed among four main sectors, including industry, building, transportation, and agriculture. The most important part of the energy strategy of a country is energy saving.4–6 Because of the limited energy sources and environmental pollution coming from using the fuels, energy saving has become compulsory. Insulation materials have become widely used in various fields since the beginning of the 20th century for energy saving.7–10 Among various thermal insulation materials, fibrous ceramic materials are of great interest due to their unique properties, such as excellent heat stability, low thermal conductivity, low specific heat, and high porosity, which can capture the air among the fibers to prevent heat transmission by convection and limit gaseous heat conduction by minimising collisions among gas molecules.11–14 In particular, silica nanofibrous (SNF) membranes with a low density structure exhibit a large number of internal spaces for air, which enable to improve the thermal insulation performance.Up to now, a great deal of effort has been devoted to preparation of SNF membranes, including chemical vapor deposition, hydrothermal treatment, and electrospinning techniques.15–17 Among the various approaches, electrospinning as a straightforward, versatile, and cost-effective technique has been widely employed to fabricate SNF membranes.18–20 By employed electrospinning and subsequent calcination process, various SNF membranes have been prepared, however, the brittleness of current SNF membranes significantly limits their practical applications.19–21 Although our previous study had revealed the formation of soft SNF membranes, the softness of the membranes was proved in the form of pictures. Meanwhile, the low tensile strength of the SNF membrane with small fiber diameter is still the challenging problems.22–25 The low tensile strength of the membranes should attribute to the loose and randomly nonwoven structures, and the slip of individual silica fibers along the stress direction.24 Recently, to enhance the tensile strength of SNF membranes, the synthesized fluorinated polyurethane were used to modify the membranes to inhibition of fiber slip.26 Although the tensile strength of the membranes were markedly increased to 15.2 MPa, the thermal stability of the composite membranes is less than 300 °C. Therefore, to improve the tensile strength of SNF membranes is a real challenge but worth to achieve since it would open access to prepare a large variety of robust tensile strength inorganic nanofibrous membranes.
As a continuation of our former research of the SNF membranes, in this paper, we intend to prepare novel SNF membranes with ultra-softness and enhanced tensile strength via an electrospinning technique with a sol–gel solution containing NaCl. Scheme 1 describes the synthesis pathway. The morphology, softness, and the tensile strength of the SNF membranes were investigated by tuning the calcination temperature and varying the NaCl content in precursor solution. Moreover, the mechanism on the softness of SNF membranes was discussed. Furthermore, the thermal insulation performance of SNF membranes was studied at room temperature.
Experimental
Materials
Poly(vinyl alcohol) (PVA, Mw = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Anhui Wanwei Group Co., Ltd., China), tetraethyl orthosilicate (TEOS), oxalic acid (H2C2O4), and NaCl were purchased from Lingfeng Chemical Co., Ltd., China. Pure water with a resistance of 18.2 MΩ was prepared using a Heal-Force system. All chemicals were of analytical grade and were used as received without further purification.
000, Anhui Wanwei Group Co., Ltd., China), tetraethyl orthosilicate (TEOS), oxalic acid (H2C2O4), and NaCl were purchased from Lingfeng Chemical Co., Ltd., China. Pure water with a resistance of 18.2 MΩ was prepared using a Heal-Force system. All chemicals were of analytical grade and were used as received without further purification.
Preparation of precursor solutions
A silica sol–gel was prepared using TEOS, H2C2O4, and pure water as starting materials by a sol–gel process. Firstly, the TEOS, H2C2O4, and pure water were mixed and stirred for 10 h at room temperature. The molar composition of TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pure water is 1
pure water is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.1 and pure water
8.1 and pure water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2C2O4 is 1
H2C2O4 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0023. Then, an equivalent weight of 25 wt% PVA solutions were dropped slowly into silica sol–gel and stirred at room temperature until the precursor solutions were obtained. In addition, to investigate the effect of NaCl on the SNF membranes, the precursor solutions were prepared by adding NaCl into the solutions and various NaCl–precursor solutions weight ratios of 0.1, 0.5, 1, and 2 wt%, respectively.
0.0023. Then, an equivalent weight of 25 wt% PVA solutions were dropped slowly into silica sol–gel and stirred at room temperature until the precursor solutions were obtained. In addition, to investigate the effect of NaCl on the SNF membranes, the precursor solutions were prepared by adding NaCl into the solutions and various NaCl–precursor solutions weight ratios of 0.1, 0.5, 1, and 2 wt%, respectively.
Fabrication of SNF membranes
The precursor solutions were transferred into a plastic syringe for electrospinning at 25 kV keeping a constant tip-to-collector distance of 25 cm. The setup for electrospinning was purchased from Shanghai Oriental Flying Nanotechnology Co., Ltd., China (DXES-01). The positive electrode of the high voltage power supply was attached to the metal needle tip while the grounded stainless drum was used as the collector wrapped with aluminum foil and rotated at 50 rpm. The feeding rate of the precursor solutions by the syringe pump was 1 mL h−1 throughout. In order to investigate the influence of the calcination temperature on the SNF membranes, the obtained precursor nanofibrous membranes were heated at 600, 800, 1000, and 1200 °C with a heating rate of 5 °C min−1 in air, respectively, then cooled down to room temperature. The precursor nanofibrous membranes with various contents of NaCl were calcined at 800 °C.Characterization
Morphologies of top surfaces and cross-sections of nanofibrous membranes were observed by field emission scanning electron microscopy (FE-SEM) (S-4800, Hitachi Ltd., Japan) and scanning electron microscopy (SEM) (TM3000, Hitachi Ltd., Japan), respectively. The fiber diameter and its distribution of each membrane were measured by an image analyzer (Adobe Photoshop CS2). Thermogravimetric analysis (TGA) of precursor membranes was performed on a thermogravimetric analyzer (TG209F1, NETZSCH, Germany) from 100 to 900 °C with a heating rate of 10 °C min−1 in air atmosphere. The softness of the membranes were measured on a softness tester (RRY-1000, Hangzhou Qingtong & Boke Automation Technology Co., Ltd., China). Ten specimens from each membrane were tested for softness. The tensile strength of the membranes were tested on a tensile tester (XQ-1C, Shanghai New Fiber Instrument Co., Ltd., China) with a crosshead speed of 5 mm min−1. Ten specimens from each membrane were tested for tensile behavior. The viscosity, conductivity, and surface tension of the precursor solutions were measured using a conductivity meter (FE30, Mettler-Toledo Group, Switzerland), a viscometer (SNB-1A, Shanghai Fangrui Instrument Co., Ltd., China), and a surface tension meter (QBZY-1, Shanghai Fangrui Instrument Co., Ltd., China), respectively. The thermal diffusivity of the membranes were obtained by a NETZSCH LFA 457 microflash instrument.Results and discussion
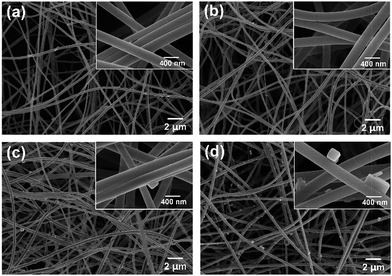
The representative FE-SEM image of electrospun precursor nanofibrous membranes revealed as randomly oriented 3D nonwoven membranes with an average diameter of 329 nm (Fig. S1 and S2, ESI†). Compared with the precursor fibrous membranes, the FE-SEM images of the SNF membranes from various calcination temperatures are shown in Fig. 1a–d, which reveal that the morphology and softness greatly change with increasing temperature. It can be seen from the inset of Fig. 1a, the SNF membranes with calcination temperature of 600 °C show light yellow, which could be attributed to the carbon distribution in the membranes by the incomplete decomposition of the precursor membranes. The phenomenon could be explained by the TGA curve and area–temperature curve (Fig. S3, ESI†), which was perfect agreement with the previously observation.24 The decomposition process of precursor membranes starts from 100 °C and ends to 700 °C, a total weight loss of 45% and area loss of 50% are observed, which was confirmed the carbon distribution of SNF membranes with calcination temperature of 600 °C.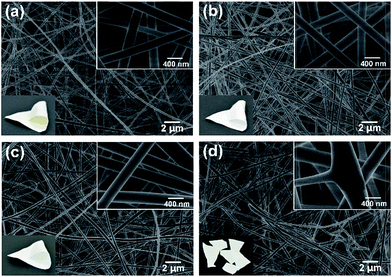 | ||
| Fig. 1 FE-SEM images of SNF membranes with various calcination temperatures of (a) 600 °C, (b) 800 °C, (c) 1000 °C, and (d) 1200 °C, respectively. Insets show the corresponding optical images. | ||
As the calcination temperature increased from 600 to 1000 °C, the SNF membranes still maintained softness and a randomly oriented fiber morphology with a fiber diameter of 200–300 nm (Fig. S2, ESI†). When the temperature up to 1200 °C, obvious melting phenomenon and an increase in fiber diameter (322 nm) could be observed, meanwhile, the membranes became fragile during bending process, which was shown in the inset of Fig. 1d. The brittleness of the SNF membranes could be caused by the melt structures between the fibers. The phenomenon reveals that the fiber morphology plays the important role in determining the softness of SNF membranes. To confirm the optimum calcination temperature, the softness and tensile strength of the SNF membranes with various calcination temperature were measured. As seen from Fig. 2a, the membranes with calcination temperature of 800 °C showed the best softness of 34.9 mN than other membranes, which should due to the smallest fiber diameter (Fig. S2, ESI†). The tensile stress–strain curves of various SNF membranes were presented in Fig. 2b. The membranes fabricated from calcination temperatures of 600 °C and 800 °C showed the typical slip phenomenon of SNF membranes during the fracture process. The SNF membranes with calcination temperature of 800 °C showed the higher tensile strength of 3.2 MPa than the other membranes. Consequently, 800 °C was selected as the most suitable calcination temperature for SNF membranes.
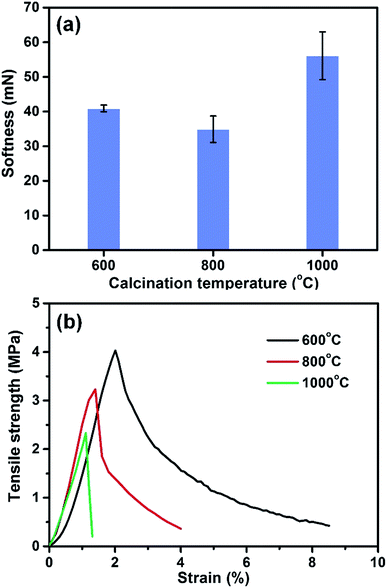 | ||
| Fig. 2 (a) Softness and (b) typical stress–strain curves of the SNF membranes with various calcination temperatures of 600, 800, and 1000 °C respectively. | ||
Substantial studies have reported that NaCl could act as an efficient element to decrease the fiber diameter,27,28 however, no previous study has shown that the NaCl had an effect on enhance the tensile strength of SNF membranes. As shown in Fig. 3a and b, the precursor fibers with the NaCl content of 0.1 wt% and 0.5 wt% exhibited the similar morphology and smaller fiber diameter of 260 nm (Fig. S4, ESI†) than the precursor fibers without NaCl (329 nm) (Fig. S1 and S2, ESI†). This phenomenon could be a consequence of the obvious increase of conductivity of the precursor solution (Fig. 4), which could greatly enhance the instability of Taylor cone during electrospinning.28–30 Further increasing the NaCl content to 1 wt% and 2 wt%, many NaCl crystals can be clearly seen on the precursor fibers (Fig. 3c and d), which is thought to be caused by solvent of the precursor solution with high NaCl content evaporation and the NaCl crystals are deposited on the fibers during the flight inside the high electric field during electrospinning, meanwhile, an increase in fiber diameter of fibers (Fig. S4, ESI†) can be corresponding to the slightly increase of viscosity of the precursor solutions (Fig. 4), respectively.
 | ||
| Fig. 3 FE-SEM images of the precursor nanofibrous membranes with various NaCl concentrations of (a) 0.1 wt%, (b) 0.5 wt%, (c) 1 wt%, and (d) 2 wt%, respectively. | ||
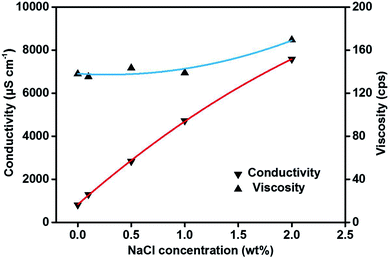 | ||
| Fig. 4 The conductivity and viscosity of the precursor solutions with various NaCl concentrations of 0, 0.1, 0.5, 1, and 2 wt%. | ||
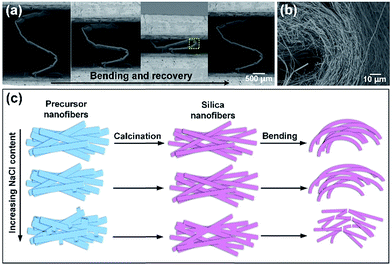
After calcination at 800 °C, the representative FE-SEM images of the SNF membranes obtained by varying the NaCl content in precursor solution are shown in Fig. 5a–d, revealing the remarkable changes in the bonding structure of relevant nanofibers. The membranes with low NaCl content (0.1 and 0.5 wt%) still maintained the softness, which exhibited similar morphology with SNF membranes without NaCl and the smaller fiber diameter (171 and 208 nm) than silica nanofibers (238 nm) in Fig. S5 in the ESI,† which was also due to the large conductivity. Further increasing the NaCl content to 1 wt%, the membranes still have softness, meanwhile, the significantly enhanced the bonding structure can be clear seen, which is marked with the yellow circle in the inset of Fig. 5c. The remarkable bonding structure between fibers can be clear seen, which should attribute to the melt phenomenon of the NaCl crystals between fibers above the melt point of 750–800 °C.31 When the NaCl content up to 2 wt%, the membranes with the largest bonding structure than the others became brittleness, which suggested that there are no proportional relation between NaCl content and softness.
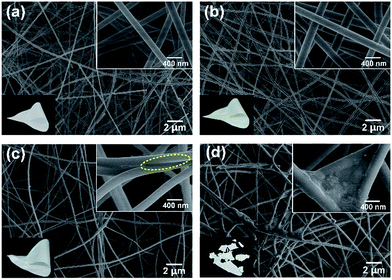 | ||
| Fig. 5 FE-SEM images of the SNF membranes with various NaCl concentrations of (a) 0.1, (b) 0.5, (c) 1, and (d) 2 wt%, respectively. Insets show the corresponding optical images. | ||
To examine the effect of NaCl on the mechanical properties, the softness and tensile strength of the SNF membranes with various NaCl contents were measured, respectively. As seen from Fig. 6 that the SNF membranes with NaCl of 0.1 wt% showed the best softness of 29 mN and the worst tensile strength of 3.9 MPa, which should attributed to the smallest fiber diameter of 171 nm than other membranes. Meanwhile, the SNF membranes with NaCl content of 1 wt% showed the highest tensile strength of 5.5 MPa and the worst softness of 40 mN, respectively. The stress–strain curve of the membranes show that the bonding structure remarked inhibited the slip phenomenon among the fibers and enhanced the tensile strength. It is difficult to simultaneously improve the softness and tensile strength of SNF membranes using NaCl as an additive. Although the diameter of the fibers with NaCl content of 1 wt% (228 nm) is smaller than pure silica nanofibers (238 nm) and even better than silk (110 mN), and the tensile strength still obvious larger than the SNF membrane without NaCl (3.2 MPa) (Fig. S5, ESI†).
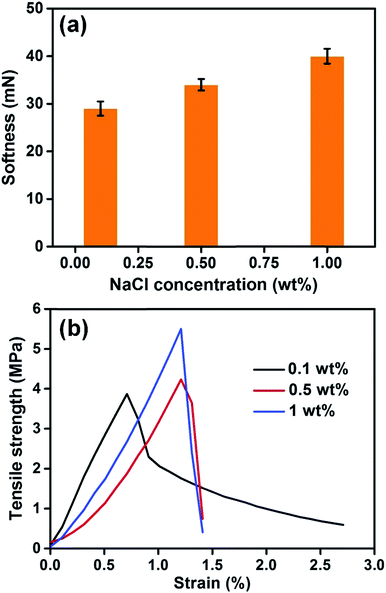 | ||
| Fig. 6 (a) Softness and (b) typical stress–strain curves of the SNF membranes with various NaCl concentrations of 0.1, 0.5, and 1 wt%. | ||
To further study the soft mechanism of the SNF membranes, in situ SEM analysis were used to evaluate the morphology of the membranes and nanofibers during the bending and recovery process. Fig. 7a presented the SEM images of the facile bending and recovering process of the membranes with NaCl content of 1 wt% revealing excellent softness with no cracks appearing during the process. Moreover, the SEM image of maximum deformation of the membranes (yellow dashed box in Fig. 7a) show the fibers are all bent in Fig. 7b. This observation is unexpected, because inorganic nanofibrous membranes and nanofibers are typically brittle.19,20 The present softness can be explained by the slip and bending of the nanofibers during bending process. Fig. 7c shows that proposal mechanism on the softness of SNF membranes. For the SNF membranes without NaCl and SNF membranes with low NaCl contents (0.1 and 0.5 wt%), the nanofibers with the relatively independent, which can slip and bent freely during the bending and recovery process (Fig. S6c and S6e, ESI†). Therefore, the membranes and nanofibers showed ultra-softness. Further increasing the NaCl content to 1 wt%, the small bonding structure was formed between nanofibers. Although the bonding structure inhibited the slip behavior between nanofibers, the softness of the membranes still maintain. However, further increasing the NaCl content to 2 wt%, careful examination of the corresponding FE-SEM image (Fig. 5d) reveals the large bonding structure obviously inhibited the slip behavior and bending of the nanofibers, which leading to the brittleness of the membranes and broken of the nanofibers (Fig. S6g, ESI†). Consequently, there are no proportional relation between the bonding structure and mechanical properties.
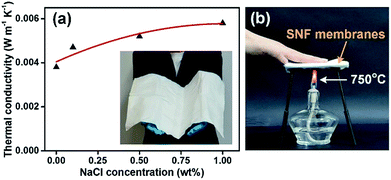
As expected, the SNF membranes with ultra-softness and robust tensile strength showed excellent thermal insulation in Fig. 8a. We tested the thermal diffusivity of the membranes by a flash diffusivity method, and the thermal conductivity was calculated from κ = ρCpD with the density (ρ), the specific heat (Cp), and the thermal diffusivity (D).32 In comparison, the thermal conductivity of the SNF membranes without NaCl was also tested, which show ultra-low thermal conductivity of 0.0038 W m−1 K−1 at room temperature and even lower than air (0.023 W m−1 K−1).33,34 Here, the ultra-low porosity in the membranes (low density of 150 kg m−3). More interestingly, the introduction of NaCl slightly improved the thermal conductivity and decreased the thermal insulation of the membranes. The phenomenon can be explained by the NaCl component between silica nanofibers, which have high specific heat and thermal conductivity belonged to crystals.35 More important, the SNF membranes can be scaled up to 60 cm × 60 cm (inset of Fig. 8a), which is of great importance for real applications. Fig. 8b presented a simple example demonstrating the excellent thermal insulation of the membranes. A hand can easily put on the membranes heating at 750 °C, suggesting a promising materials for a wide range of potential applications in bunker clothing and thermal insulation materials in aerospace.
Conclusions
In summary, the SNF membranes with ultra-softness and enhanced tensile strength have been successfully developed via an electrospinning process using NaCl as an additive. The calcination temperature and NaCl content in precursor solution played an important role in the morphology, softness, and tensile strength of the SNF membrane. The SNF membranes with the NaCl content of 1 wt% exhibited the relative high tensile strength of 5.5 MPa, and the better softness of 40 mN than silk (110 mN). Additionally, in situ SEM analysis has indicated the proposed mechanism on the softness of the SNF membranes, revealing the slip between nanofibers and bending behavior of the nanofibers during the bending and recovery process. Moreover, the SNF membranes with NaCl content of 1 wt% exhibited ultra-low thermal conductivity of 0.0058 W m−1 K−1 and even lower than air, which make them a good candidate to be useful in bunker clothing. This work also provided a versatile strategy for further design and development of other soft ceramic nanofibrous membranes towards various applications.Acknowledgements
This work is supported by the National Natural Science Foundation of China (no. 51322304 and U1232116), the Fundamental Research Funds for the Central Universities, and the “DHU Distinguished Young Professor Program”.Notes and references
- H. Niu, Y. He, U. Desideri, P. Zhang, H. Qin and S. Wang, Renewable Energy, 2014, 65, 137–145 CrossRef PubMed.
- J. You, Renewable Sustainable Energy Rev., 2011, 15, 2984–2989 CrossRef PubMed.
- K. Jayanthakumaran, R. Verma and Y. Liu, Energy Policy, 2012, 42, 450–460 CrossRef PubMed.
- H. Li, H. Ogle, B. Jiang, M. Hagar and B. Li, J. Orthop. Res., 2010, 28, 992–999 CrossRef CAS PubMed.
- C. Chiou, C. Chiou, C. Chu and S. Lin, Energy and Buildings, 2008, 40, 1660–1665 CrossRef PubMed.
- Y. Chang, Energy and Buildings, 2007, 39, 437–444 CrossRef PubMed.
- F. Wang, J. Liang, Q. Tang, C. Chen and Y. Chen, J. Nanosci. Nanotechnol., 2014, 14, 3861–3867 CrossRef CAS PubMed.
- V. Peletskii and B. Shur, Refract. Ind. Ceram., 2007, 48, 356–358 CrossRef CAS PubMed.
- C. Sheng, Y. Yu, Y. Yu, L. Mi, G. Tang and L. Song, J. Inorg. Mater., 2013, 28, 790–794 CAS.
- Y. Shih, F. Cheung, J. Koo and B. Yang, J. Thermophys. Heat Transfer, 2003, 17, 53–61 CrossRef CAS PubMed.
- M. Schulte, R. Klima, R. Bredehoft and W. Beier, Stahl Eisen, 1988, 108, 553–558 CAS.
- R. Ji, Z. Zhang, L. Liu and X. Wang, Text. Res. J., 2014, 84, 411–421 CrossRef CAS PubMed.
- J. Marschall and F. Milos, J. Thermophys. Heat Transfer, 1998, 12, 528–535 CrossRef CAS.
- N. Du, J. Fan and H. Wu, Fibers Polym., 2008, 9, 27–33 CrossRef PubMed.
- S. An, B. N. Joshi, M. W. Lee, N. Y. Kim and S. S. Yoon, Appl. Surf. Sci., 2014, 294, 24–28 CrossRef CAS PubMed.
- N. Gougeon, J. Sangleboeuf, R. El Abdi, M. Poulain and C. Tistere-Borda, Fiber Integr. Opt., 2005, 24, 491–500 CrossRef CAS.
- M. Xi, X. Wang, Y. Zhao, Q. Feng, F. Zheng, Z. Zhu and H. Fong, Mater. Lett., 2014, 120, 219–223 CrossRef CAS PubMed.
- H. Kim, C. Shao, J. Gong, B. Ding, D. Lee and S. Park, Mater. Lett., 2003, 57, 1579–1584 CrossRef.
- A. Katoch and S. Kim, J. Am. Ceram. Soc., 2012, 95, 553–556 CrossRef CAS PubMed.
- J. Saha and G. De, Chem. Commun., 2013, 49, 6322–6324 RSC.
- Y. Wu, F. Li, Y. Wu, W. Jia, P. Hannam, J. Qiao and G. Li, Colloid Polym. Sci., 2011, 289, 1253–1260 CAS.
- M. Guo, B. Ding, X. Li, X. Wang, J. Yu and M. Wang, J. Phys. Chem. C, 2010, 114, 916–921 CAS.
- F. Zhao, X. Wang, B. Ding, J. Lin, J. Hu, Y. Si, J. Yu and G. Sun, RSC Adv., 2011, 1, 1482–1488 RSC.
- X. Mao, Y. Si, Y. Chen, L. Yang, F. Zhao, B. Ding and J. Yu, RSC Adv., 2012, 2, 12216–12223 RSC.
- L. Yang, A. Raza, Y. Si, X. Mao, Y. Shang, B. Ding, J. Yu and S. Al-Deyab, Nanoscale, 2012, 4, 6581–6587 RSC.
- X. Mao, Y. Chen, Y. Si, Y. Li, H. Wan, J. Yu, G. Sun and B. Ding, RSC Adv., 2013, 3, 7562–7569 RSC.
- N. Wang, X. Wang, B. Ding, J. Yu and G. Sun, J. Mater. Chem., 2012, 22, 1445–1452 RSC.
- X. Wang, B. Ding, G. Sun, M. Wang and J. Yu, Prog. Mater. Sci., 2013, 58, 1173–1243 CrossRef CAS PubMed.
- R. Friend, R. Gymer, A. Holmes, J. Burroughes, R. Marks, C. Taliani, D. Bradley, D. Dos Santos, J. Bredas, M. Logdlund and W. Salaneck, Nature, 1999, 397, 121–128 CrossRef CAS PubMed.
- A. Holzmeister, A. Yarin and J. Wendorff, Polymer, 2010, 51, 2769–2778 CrossRef CAS PubMed.
- G. Breaux, R. Benirschke and M. Jarrold, J. Chem. Phys., 2004, 121, 6502–6507 CrossRef CAS PubMed.
- S. Min, J. Blumm and A. Lindemann, Thermochim. Acta, 2007, 455, 46–49 CrossRef CAS PubMed.
- P. Gaal, M. Thermitus and D. Stroe, J. Therm. Anal. Calorim., 2004, 78, 185–189 CrossRef CAS.
- S. Beirao, A. Ribeiro, M. Lourenco, F. Santos and C. de Castro, Int. J. Thermophys., 2012, 33, 1686–1703 CrossRef CAS PubMed.
- H. Shibata, Y. Waseda, H. Ohta, K. Kiyomi, K. Shimoyama, K. Fujito, H. Nagaoka, Y. Kagamitani, R. Simura and T. Fukuda, Mater. Trans., 2007, 48, 2782–2786 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: FE-SEM image of electropun precursor nanofibrous membranes is shown in Fig. S1. The average diameter of electropun precursor nanofibers and silica nanofibers with various calcination temperatures of 600, 800, 1000, and 1200 °C are shown in Fig. S2. Fig. S3 displays thermogravimetric analysis of precursor nanofibrous membranes and the area of the membranes from precursor to SNF membranes with calcination temperatures of 600, 800, 1000, and 1200 °C, respectively. Fig. S4 shows the average diameter of the precursor nanofibers with various NaCl concentrations of 0.1, 0.5, 1, and 2 wt%. Fig. S5 shows the average diameter of silica nanofibers with various NaCl concentrations of 0.1, 0.5, 1, and 2 wt%. Fig. S6 shows the SEM images of SNF membranes with various NaCl concentrations of 0, 0.1, 0.5, and 2 wt% during the bending process. See DOI: 10.1039/c4ra12271b |
| ‡ These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |