Poly(benzo[2,1-b:3,4-b′]dithiophene-alt-isoindigo): a low bandgap polymer showing a high open circuit voltage in polymer solar cells†
Meixiu Wanabc,
Hongbing Zhuc,
Juan Liuab and
Lijun Huo*ab
aKey Laboratory of Bio-inspired Smart Interfacial Science and Technology of Ministry of Education, Beijing Key Laboratory of Bio-inspired Energy Materials and Devices, School of Chemistry and Environment, Beihang University, Beijing 100191, P. R. China. E-mail: huolijun@buaa.edu.cn
bHeeger Beijing Research and Development Center, School of Chemistry and Environment, Beihang University, Beijing 100191, P. R. China
cDepartment of Chemistry and Material Science, Hunan Institute of Humanities, Science and Technology, Loudi 417000, P. R. China
First published on 18th November 2014
Abstract
A novel optical band gap of 1.57 eV isoindigo (IID)-containing polymer of PBDP-IID was synthesized and the HOMO of the polymer descends to −5.44 eV due to strong electron-deficient isoindigo unit. Bulk heterojunction solar cells made from PBDP-IID exhibited a high Voc of 0.95 V, which shows the highest value among the PSCs based on the narrow bandgap polymer donors with Eg < 1.6 eV.
Low band gap (Eg) conjugated polymer photovoltaic donor materials have been a hot research subject in the field of polymer solar cells (PSCs) in recent years, owing to their distinguished optical and optoelectronic response to visible and NIR light.1 Compared to poly(3-hexylthiophene) (P3HT) which is the most representative polymer donor material, more photons can be absorbed from the low band gap polymers and transferred into effective currents. Thus developing low band gap polymers has become an effective strategy to realize highly efficient PSCs.2 The low bandgap conjugated polymers can be obtained by copolymerization of an electron-donating unit (D) and an electron-accepting unit (A),3,4 attaching electron-rich or electron-deficient functional substituents, or using quinoid resonance structure.2 However, lowering Eg often results in a high-lying HOMO energy level of the conjugated polymers,5 which will make the polymer unstable against oxidation and lead to a lower open circuit voltage (Voc) of the PSCs based on the polymer as donor. Therefore, in the molecular design of the photovoltaic donor polymers, decreasing the HOMO and LUMO energy levels whilst keeping Eg low should be considered.
Isoindigo, known since antiquity, is industrially used as dyestuff,6 and now it is also found important photovoltaic effect.7 As shown in Scheme 1, isoindigo bears two symmetrical oxindole motif and can be regarded as a compound with trans stilbene hydrocarbon backbone linked by two lactame rings. The two lactame rings affiliated to adjacent aryl rings in isoindigo ensure its planar molecular structure. Recently, it has been reported that isoindigo is obviously a strong electron-accepting unit.7 Therefore, isoindigo could be a promising acceptor unit in D–A copolymers with low bandgap and lower LUMO energy level.7d–j In the other hand, benzo[2,1-b:3,4-b′]dithiophene (BDP) as a derivative of benzo[1,2-b:4,5-b′]dithiophene (BDT) is modified by a benzo-annellation. From molecular simulations and experiments results in some papers, the HOMO levels of homopolymers of BDP and BDT are −5.70 eV and −5.16 eV, respectively.8 Obviously, the former exhibits a lower HOMO level than that of the former, which suggests that the a new polymer containing BDP unit can show a lower-lying HOMO than those similar structure based BDT polymers. Meanwhile, BDP containing polymers have showed high photovoltaic performances.8–10
Based on these considerations, in this work we synthesized a new low band gap D–A copolymer of poly(benzo[2,1-b:3,4-b′]dithiophene-alt-isoindigo) (PBDP-IID) with BDP as the donor unit and isoindigo as a acceptor unit. PBDP-IID possesses a low Eg of 1.57 eV and a lower HOMO energy level of −5.44 eV. The PSCs based PBDP-IID as donor demonstrated a power conversion efficiency of 5.07% with a high open circuit voltage (Voc) of 0.95 V. To our knowledge, the Voc of 0.95 V is the highest value among the PSCs based on the narrow bandgap polymer donors with Eg < 1.6 eV.
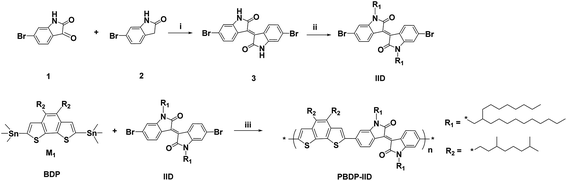
The synthetic route of PBDP-IID is shown in Scheme 2. In acidic condition, 6-bromoisatin (1) and 6-bromooxindole (2) was condensed to form 1,1′-dihydro-6,6′-dibromoisoindigo (3) as brown solid power with 90% yield. Then alkyl side chains was employed to increase the solubility of isoindigo. The monomer of isoindigo was copolymerized with the monomer of BDP unit by typical Stille coupling reaction to obtain deep dark green solid power PBDP-IID with a 64% yield.
 | ||
| Scheme 2 Synthetic route of PBDP-IID: (i) acetic acid, conc. hydrochloric acid, reflux, 9 h; (ii) 2-ethylhexyl bromide, 12 h, DMF, K2CO3 100 °C, 10 h, argon; (iii) Pd(PPh3)4, toluene, reflux, 20 h. | ||
The polymer of PBDP-IID is readily dissolved in common solvents such as chloroform and THF. The corresponding weight average molecular weight (Mw) of PBDP-IID is 37.6 K, with a polydispersity index (PDI) of 2.3. Thermal stability of the polymer was investigated with thermogravimetric analysis (TGA) (see ESI†). The TGA result reveals that, in the air, the onset temperature with 5% weight-loss (Td) of PBDP-IID is 379 °C. This indicates that it has good thermal stability against oxygen, which is very important in device fabrication process and other kinds of applications.
Fig. 1 shows the absorption spectra of PBDP-IID in dilute chloroform solutions (s) and solid thin films (f). The absorption spectrum in dilute chloroform shows two absorption bands, which are common characters for D–A type low band gap polymers. The absorption band located at 300–500 nm should be attributed to the π–π* transition of the conjugated polymer main chains and the longer wavelength absorption band located at 500–800 nm are caused by intramolecular charge transfer (ICT) interaction between electron-rich BDP and electron-deficient IID units. The main absorption peak and onset of PBDP-IID appeared at 678 nm and 769 nm, respectively. In solid film state, the absorption spectrum of PBDP-IID shows red shift in some degree. The main absorption peak is red shifted to 691 nm and absorption edge extended to 792 nm due to more aggregated configurations formed in film than in solution. The optical bandgaps (Eoptg) calculated from the absorption edge of the PBDP-IID film is 1.57 eV, which confirms that the low band gap conjugated polymer is indeed realized by incorporating new isoindigo acceptor unit in the D–A copolymers.
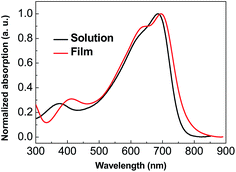 | ||
| Fig. 1 Normalized UV-Vis absorption spectra of PBDP-IID in dilute CHCl3 solutions (s) and in solid films (f). | ||
Electrochemical cyclic voltammetry has been widely employed to investigate the redox behavior and to estimate the HOMO and LUMO energy levels of a conjugated polymer. Fig. 2 shows the cyclic voltammogram of PBDP-IID. Clearly, PBDP-IID shows reversible p-doping/dedoping and n-doping/dedoping processes in the positive and negative scanning potential range. The onset oxidation potential (Eox) is 0.73 V vs. Ag/Ag+ and the onset reduction potential (Eox) is −0.94 V vs. Ag/Ag+, respectively. The corresponding HOMO and LUMO values are calculated according to the equations LUMO = −e(Ered + 4.71) (eV) and HOMO = −e(Eox + 4.71) (eV)11 (where the units of Eox and Ered are V vs. Ag/Ag+). The corresponding HOMO and LUMO values are −5.44 eV and −3.77 eV, respectively. The electrochemical band gap (Eecg), taken as the difference between the onsets of oxidation and reduction potentials, is equal to 1.67 eV for PBDP-IID. The deeper HOMO level of PBDP-IID is favorable for realizing high open-circuit voltage (Voc) of the PSCs based on the polymer as donor.
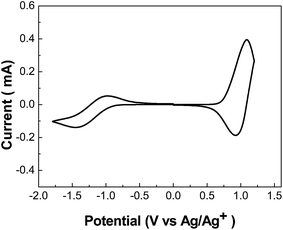 | ||
| Fig. 2 Cyclic voltammograms of the PBDP-IID film on a platinum electrode in acetonitrile solution containing 0.1 mol L−1 Bu4NPF6 at a scan rate of 20 mV s−1. | ||
To evaluate the effect of the strong electron-deficient IID unit in the copolymer with BDP unit on the photovoltaic properties, polymer solar cells (PSCs) were fabricated with a structure of ITO/PEDOT:PSS/PBDP-IID/PC70BM/Ca/Al. The active layers were spin-coated from o-dichlorobenzene solutions with different PBDP-IID/PC70BM weight ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively. The devices were completed by evaporating Ca/Al metal electrodes with an area of 4 mm2 as defined by masks. Fig. 3 shows J–V curves of the devices under illumination of AM 1.5G (100 mW cm−2) and the device performance parameters are listed in Table 1. The photovoltaic performance of PBDP-IID exhibited a remarkable high Voc of above 0.9 V for such low band gap of 1.57 eV. The Jsc increases from 6.77 mA to 9.21 mA and to 7.81 mA when the PBDP-IID/PC70BM weight ratio changes from 1
3, respectively. The devices were completed by evaporating Ca/Al metal electrodes with an area of 4 mm2 as defined by masks. Fig. 3 shows J–V curves of the devices under illumination of AM 1.5G (100 mW cm−2) and the device performance parameters are listed in Table 1. The photovoltaic performance of PBDP-IID exhibited a remarkable high Voc of above 0.9 V for such low band gap of 1.57 eV. The Jsc increases from 6.77 mA to 9.21 mA and to 7.81 mA when the PBDP-IID/PC70BM weight ratio changes from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and to 1
2 and to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, but FF changed little at about 55%. A higher power conversion efficiency (PCE) of 5.07% was obtained under the condition of 1
3, but FF changed little at about 55%. A higher power conversion efficiency (PCE) of 5.07% was obtained under the condition of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
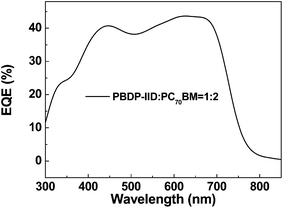
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 weight ratio. The results indicate more acceptor content in the active layer could lead to better balance between electrons and holes transfer process. Additional, the J–V curves of with different incident light intensity (from 100 to 1 mW cm−2) are measured and it was found Jsc is linear with illuminated light intensity and there is no obvious change for Voc in the devices. The FF appears maximum at 70 mW cm−2 but ultimately the optimized PCE reaches its maximum of 5.07% at 100 mW cm−2. Meanwhile, Fig. 4 shows the EQE curves of the PSC based on a blend of PBDP-IID/PCBM with a optimized weight ratio, which matches well the absorption range of the active layer blend.
2 weight ratio. The results indicate more acceptor content in the active layer could lead to better balance between electrons and holes transfer process. Additional, the J–V curves of with different incident light intensity (from 100 to 1 mW cm−2) are measured and it was found Jsc is linear with illuminated light intensity and there is no obvious change for Voc in the devices. The FF appears maximum at 70 mW cm−2 but ultimately the optimized PCE reaches its maximum of 5.07% at 100 mW cm−2. Meanwhile, Fig. 4 shows the EQE curves of the PSC based on a blend of PBDP-IID/PCBM with a optimized weight ratio, which matches well the absorption range of the active layer blend.
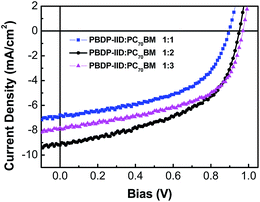 | ||
| Fig. 3 J–V curves of the polymer solar cells based on PBDP-IID/PC70BM under illumination of AM 1.5, 100 mW cm−2. | ||
| Weight ratio | Thickness (nm) | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 | 0.91 | 6.77 | 54 | 3.33 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
100 | 0.95 | 9.21 | 58 | 5.07 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
92 | 0.97 | 7.81 | 56 | 4.24 |
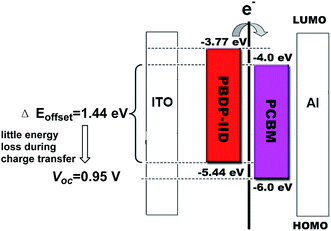
It is very interesting to note that an impressive high Voc of 0.95 V was obtained for the PSC based on this rather low band gap (<1.6 eV) polymer donor. The high Voc origination could be discussed from the energy level chart shown in Fig. 5. Considering a charge separation drive force of about 0.2–0.3 eV for the exciton dissociation from the LUMO offset of PBDP-IID and PC70BM, there is only 1.44 eV left between the HOMO of PBDP-IID and the LUMO of PC70BM. According to the theory equation: Voc = |Edonor HOMO| − |Eacceptor LUMO| − 0.3 V,12 little energy loss during the charge transfer and lead to a very high Voc of 0.95 V. Obviously, the high Voc originates from the low-lying HOMO of the PBDP-IID donor material.
Morphology study
Surface morphology of the blend films of polymers/PC70BM with different weight ratios were investigated by the atomic force microscope (AFM). As shown in Fig. 6(a) and (c), there is very large domain sizes between D/A interfacial areas, which makes the surface morphology rather roughness. In Fig. 6(b), on the contrary, the domain sizes becomes smaller and surface becomes smoother than that in Fig. 6(a) and (c). From our measurement results, root-meansquare (RMS) of these films are 6.6 nm, 2.1 nm and 3.1 nm for AFM height images with different weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively, which coincides above conclusions. These very different morphologies imply that the different weight ratio for polymer/PCBM blends affect the interactions between D/A interfacial areas and at a ratio of 1
3, respectively, which coincides above conclusions. These very different morphologies imply that the different weight ratio for polymer/PCBM blends affect the interactions between D/A interfacial areas and at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 condition the morphology has been better optimized. Therefore, it is reasonable to get the highest short circuit current and PCE from a blend of 1
2 condition the morphology has been better optimized. Therefore, it is reasonable to get the highest short circuit current and PCE from a blend of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 weight ratio for PBDP-IID based devices.
2 weight ratio for PBDP-IID based devices.
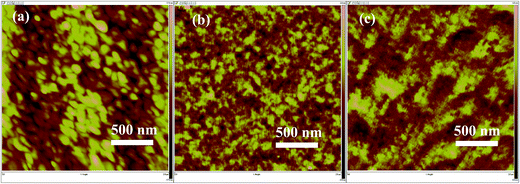 | ||
Fig. 6 AFM height images (2 × 2 μm2) of the active layers from polymer:PC70BM with different weight ratio of (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (b) 1 1; (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; (c) 1 2; (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. 3. | ||
In summary, a new low band gap D–A copolymer of PBDP-IID containing BDP donor unit and IID acceptor unit was synthesized for the application as donor materials in PSCs. The polymer showed a low optical band gap of 1.57 eV and possess a low-lying HOMO energy level of −5.44 eV. The PSCs made from blends of this polymer and PC70BM exhibited an efficient PCE of 5.07% a high Voc of 0.95 V, which is the highest value reported so far for the PSCs based on the polymer donors with a band gap below 1.6 eV. The low-lying HOMO energy level of PBDP-IID plays a main role in determining this high Voc. The results indicate that the isoindigo-based D–A copolymer could possess a narrow bandgap as well as low-lying HOMO level. Therefore it shows a promising potential for the PSCs with tandem structures.
Acknowledgements
This work was supported by National Natural Science Foundation of China (NSFC) (no. 51273203 and 51261160496).References
- (a) Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868 CrossRef CAS PubMed; (b) J. W. Chen and Y. Cao, Acc. Chem. Res., 2009, 42, 1709 CrossRef CAS PubMed; (c) Y. F. Li, Acc. Chem. Res., 2012, 45, 723 CrossRef CAS PubMed; (d) L. J. Huo and J. H. Hou, Polym. Chem., 2011, 2, 2453 RSC.
- (a) H. Y. Chen, J. H. Hou, S. Q. Zhang, Y. Y. Liang, G. W. Yang, Y. Yang, L. P. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649 CrossRef CAS; (b) L. J. Huo, H. Y. Chen, J. H. Hou, T. L. Chen and Y. Yang, Chem. Commun., 2009, 5570 RSC; (c) Y. Y. Liang, D. Q. Feng, Y. Wu, S. T. Tsai, G. Li, C. Ray and L. P. Yu, J. Am. Chem. Soc., 2009, 131, 7792 CrossRef CAS PubMed; (d) Y. Y. Liang and L. P. Yu, Acc. Chem. Res., 2010, 43, 1227 CrossRef CAS PubMed; (e) L. J. Huo, Z. Li, X. Guo, Y. Wu, M. J. Zhang, L. Ye, S. Zhang and J. H. Hou, Polym. Chem., 2013, 4, 3047 RSC.
- J. Roncali, Chem. Rev., 1997, 97, 173 CrossRef CAS PubMed.
- (a) Q. T. Zhang and J. M. Tour, J. Am. Chem. Soc., 1998, 120, 5355 CrossRef CAS; (b) L. J. Huo, X. Guo, S. Q. Zhang, Y. F. Li and J. H. Hou, Macromolecules, 2011, 44, 4035 CrossRef CAS; (c) L. J. Huo, L. Ye, Y. Wu, Z. J. Li, X. Guo, M. J. Zhng, S. Q. Zhang and J. H. Hou, Macromolecules, 2012, 45, 6923 CrossRef CAS; (d) Y. Wu, Z. Li, X. Guo, H. Fan, L. J. Huo and J. H. Hou, J. Mater. Chem., 2012, 22, 21362 RSC; (e) X. Guo, M. J. Zhang, L. J. Huo and J. H. Hou, J. Mater. Chem., 2012, 22, 21024 RSC; (f) Y. Wu, Z. J. Li, W. Ma, Y. Huang, L. J. Huo, X. Guo, M. J. Zhang, H. Ade and J. H. Hou, Adv. Mater., 2013, 25, 3449 CrossRef CAS PubMed.
- C. Shi, Y. Yao, Y. Yang and Q. Pei, J. Am. Chem. Soc., 2006, 128, 8980 CrossRef CAS PubMed.
- J. F. M. Silva, S. J. Garden and A. C. Pinto, J. Braz. Chem. Soc., 2001, 12, 273 CrossRef PubMed.
- (a) J. G. Mei, K. R. Graham, R. Stalder and J. R. Reynolds, Org. Lett., 2010, 12, 660 CrossRef CAS PubMed; (b) R. Stalder, J. G. Mei and J. R. Reynolds, Macromolecules, 2010, 43, 8348 CrossRef CAS; (c) G. Zang, Y. Fu, Z. Xie and Q. Zhang, Macromolecules, 2011, 44, 1414 CrossRef; (d) B. Liu, Y. Zou, B. Peng, B. Zhao, K. Huang, Y. He and C. Pan, Polym. Chem., 2011, 2, 1156 RSC; (e) E. Wang, Z. Ma, Z. Zhang, P. Henriksson, O. Inganäs, F. Zhang and M. R. Andersson, Chem. Commun., 2011, 47, 4908 RSC; (f) T. Lei, Y. Cao, Y. Fan, C. Liu, S.-C. Yuan and J. Pei, J. Am. Chem. Soc., 2011, 133, 6099 CrossRef CAS PubMed; (g) Z. Ma, D. Dang, Z. Tang, D. Gedefaw, J. Bergqvist, W. Zhu, W. Mammo, M. R. Andersson, O. Inganäs, F. Zhang and E. Wang, Adv. Energy Mater., 2014, 4, 22 Search PubMed; (h) Y. Deng, J. Liu, J. Wang, J. Liu, W. Tian, H. Zhang, X. Xie, Y. Geng and F. Wang, Adv. Mater., 2014, 26, 471 CrossRef CAS PubMed; (i) E. Wang, Z. Ma, Z. Zhang, K. Vandewal, P. Henriksson, O. Inganäs, F. Zhang and M. R. Andersson, J. Am. Chem. Soc., 2011, 133, 14244 CrossRef CAS PubMed; (j) Z. Ma, D. Dang, Z. Tang, D. Gedefaw, J. Bergqvist, W. Zhu, W. Mammo, M. R. Andersson, O. Inganäs, F. Zhang and E. Wang, Adv. Energy Mater., 2014, 4, 1301455 Search PubMed.
- (a) S. Q. Xiao, H. X. Zhou and W. You, Macromolecules, 2008, 41, 5688 CrossRef CAS; (b) S. Q. Xiao, A. C. Stuart, S. Liu and W. You, ACS Appl. Mater. Interfaces, 2009, 1, 1613 CrossRef CAS PubMed; (c) H. Zhou, L. Yang, S. Price, K. Knight and W. You, Angew. Chem., Int. Ed., 2010, 49, 7992 CrossRef CAS PubMed.
- L. J. Huo, J. H. Hou, H. Y. Chen, S. Q. Zhang, Y. Jiang, T. L. Chen and Y. Yang, Macromolecules, 2009, 42, 6564 CrossRef CAS.
- L. J. Huo, X. Guo, Y. F. Li and J. H. Hou, Chem. Commun., 2011, 47, 8850 RSC.
- (a) J. H. Hou, Z. Tan, Y. Yan, Y. J. He, C. H. Yang and Y. F. Li, J. Am. Chem. Soc., 2006, 128, 4911 CrossRef CAS PubMed; (b) Y. F. Li, Y. Cao, J. Gao, D. L. Wang, G. Yu and A. J. Heeger, Synth. Met., 1999, 99, 243 CrossRef CAS; (c) Q. J. Sun, H. Q. Wang, C. H. Yang and Y. F. Li, J. Mater. Chem., 2003, 13, 800 RSC; (d) Y. J. He, H. Y. Chen, J. H. Hou and Y. F. Li, J. Am. Chem. Soc., 2010, 132, 1377 CrossRef CAS PubMed.
- M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra12171f |
| This journal is © The Royal Society of Chemistry 2015 |