Metal–elastomer nanostructures for tunable SERS and easy microfluidic integration†
Abstract
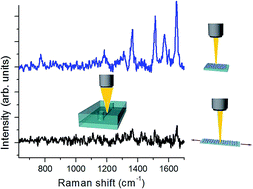
Stretchable plasmonic nanostructures constituted by Ag nanoparticles on flexible elastomeric matrices are synthesized and used as surface-enhanced Raman scattering (SERS) substrates. The structure consists of silver particles deposited by DC sputtering on polydimethylsiloxane (PDMS). The optical transmittance spectra show marked dips related to plasmonic inter-particle short-range interactions. The substrates show noticeable Raman enhancement allowing detection of R6G at very low concentration. Under mechanically controlled stretching, the interparticle gap sizes change yielding a reversible spectral shift of the plasmonic resonance. Experimental results are validated by 3D modeling. When such a resonance matches the Raman excitation line, pronounced enhancements can be achieved, optimizing the SERS regime. Taking advantage of the PDMS matrices, these tunable SERS-active substrates are integrated in microfluidic circuitry fruitfully exploitable for on-chip label-free detection.
- This article is part of the themed collection: Surface enhanced Raman Spectroscopy: Editors collection for RSC Advances

 Please wait while we load your content...
Please wait while we load your content...