Nanometer-level high-accuracy molding using a photo-curable silicone elastomer by suppressing thermal shrinkage
Abstract
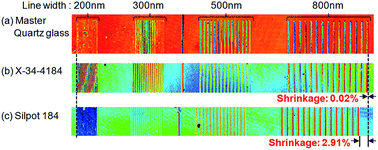
Although the so-called “labs-on-a-chip” or micro total analysis systems (micro TAS) fields hold high promise for applications in many fields, conventional fabrication processes based on the semiconductor industry such as photolithography have limitations in terms of productivity. Silicone elastomers are widely used for micromodeling and offer biocompatibility and chemical stability, but they are generally thermosetting and undergo unacceptable levels of shape deformation during curing. In this study, a photocurable silicone elastomer that has recently become commercially available was examined, and its basic optical, mechanical, and other related characteristics, along with its shape transfer capabilities, particularly its nanostructure replication characteristics, were measured in comparison with those of a representative existing thermosetting silicone elastomer. As a result, the photo-cured elastomer was shown to be superior to existing heat-cured silicone elastomers, having mechanical strength approximately three times greater, and was shown to have the same optical transmittance, extending from the near-IR to the near-UV regions. In addition, it was shown that the elastomer is sensitive to light in a wide range of wavelengths, from 254 to 600 nm, with no large difference in its curing characteristics, indicating that curing can be performed under a variety of common forms of illumination. Most importantly, the photocured elastomer provided extremely high replication accuracy due to its thermal shrinkage of less than 0.02%, compared to 2.91% in the heat-cured elastomer.

 Please wait while we load your content...
Please wait while we load your content...