Acorus Linnaeus: a review of traditional uses, phytochemistry and neuropharmacology
Xiao-Lin Feng†
,
Yang Yu†,
Da-Peng Qin,
Hao Gao* and
Xin-Sheng Yao*
Institute of Traditional Chinese Medicine & Natural Products, College of Pharmacy, Jinan University, Guangzhou 510632, P. R. China. E-mail: tyaoxs@gmail.com; tghao@jnu.edu.cn; Fax: +86-20-85221559; Tel: +86-20-85221559
First published on 28th November 2014
Abstract
Acorus Linnaeus is a genus of perennial herbs distributed from the northern temperate to the subtropical regions, and has been widely used as traditional folk medicine in China and India since ancient times. Phytochemical studies have shown the presence of numerous beneficial compounds, such as phenylpropanoids, lignans, sesquiterpenoids, alkaloids and others. Neuropharmacological studies have revealed that the Acorus rhizome extract and its constituents, particularly α- and β-asarone, possess anticonvulsant, antiepileptic, neuroprotective, memory enhancing, and sedative properties. This review summarises the traditional uses, phytochemistry, and neuropharmacological activities of Acorus Linnaeus.
Introduction
In 1998, the Angiosperm Phylogeny Group (APG) revised the classification of Acoraceae as a monotypic family.1,2 However, Acorus has historically been considered a member of the Araceae family, although the family Acoraceae was already established in 1820.2,3 Several significant morphological, anatomical and embryological characteristics in addition to DNA evidence have supported the view that Acorus is not closely related to the Araceae at all.3,4 However, the phylogenetic relationships among the species of Acorus, which are usually divided into 2 to 5 species, remain unclear.3–5According to the traditional use and recent studies on Acorus, in this review, we follow the systematic classification established by H. Li.5 The Ggenus Acorus contains four species, A. calamus L., A. tatarinowii Schott, A. gramineus Soland and A. rumphianus S. Y. Hu, which are widely distributed in eastern and southern Asia. With the exception of A. rumphianus, the species have historically been used in traditional medicine, particularly for the treatment of central nervous system (CNS) diseases in several ancient Asian countries.5 The rhizomes of A. tatarinowii have been recorded in the Chinese Pharmacopoeia since 1985. However, the rhizomes of A. calamus are thought to be adulterants of the rhizomes of A. tatarinowii in the modern Chinese herbal medicine market.
The well-known traditional herbal use of Acorus has inspired numerous studies on their rich constituents and pharmacological activities. This comprehensive review on Acorus summarises the traditional herbal use and the phytochemicals that have currently been identified. Furthermore, a preliminary comparison between Acorus treatment of CNS diseases in traditional medicine and in pharmacological studies is presented.
Traditional uses
Primarily because of their rich content of essential oil, the rhizomes of the Acorus species have traditionally been used to treat CNS disorders, respiratory system diseases, gastrointestinal disorders and other diseases. The roots and leaves have not been as widely used because of their low oil content.3A. calamus, which is commonly known as “sweet flag” or “calamus”, is native to Central Asia and Eastern Europe.6 The ancient peoples of China used A. calamus as an emetic in dyspepsia and as a sedative, nerve tonic, antimicrobial agent, and expectorant.5 In the ayurvedic system of medicine, the rhizomes of A. calamus are thought to possess aromatic, stimulant, bitter tonic, emetic, expectorant, emmenagogue, aphrodisiac, laxative, diuretic, antispasmodic, carminative, and anthelmintic properties. A. calamus has been used for the treatment of numerous diseases, such as mental ailments, including epilepsy, schizophrenia, and memory disorders; chronic diarrhoea and dysentery; bronchial catarrh; intermittent fevers; and asthma, among others.8,9 Additionally, A. calamus has been widely used in traditional folk medicine in America and Indonesia for gastrointestinal disorders, such as colic pain, diarrhoea and the radix in the therapy of diabetes.3
A. tatarinowii (Shi-chang-pu in Chinese), recorded in “Shen Nong's Herbal Classic”, is a famous traditional Chinese medicine (TCM) for treating CNS diseases. In “Shen Nong's Herbal Classic”, A. tatarinowii belongs to the superior medicinal herbs because of its ability to prolong life. The dried rhizomes of A. tatarinowii have been conventionally prescribed by traditional Chinese doctors to cure difficult diseases, such as epilepsy, amnesia, apoplexy and dementia, or in combination with other medicinal herbs for the improvement of learning and memory.5,10
A. gramineus, which is also known as “Japanese sweet flag”, has historically been used for the treatment of cognitive decline, bronchial catarrh, stomach ache, edema and as an insecticide.5,11 A. rumphianus has not been used to treat diseases because of its rare distribution.
Phytochemistry
Over the past fifteen years, studies on bioactive phytochemicals from Acorus species have significantly increased, and an increasing number of bioactive constituents has been discovered and reported. The major active constituents identified were α- and β-asarone (1 and 2) (Fig. 1), to which most of the bioactivities of the Acorus species were attributed.121. Phenylpropanoids
The most notable constituents of Acorus oil are α- and β-asarone (1 and 2). Mazza13 has investigated the essential oil constituents of two varieties of A. calamus L. The primarily volatile constituents were detected as hydrocarbons, carbonyl compounds, alcohols and phenols using gas chromatography-mass spectrometry. The European essential oil substantially differed from that of the Indian variety, which was characterised by a higher β-asarone (2) content (77.68%).The compounds α- and β-asarone (1 and 2) have been reported to exhibit a wide spectrum of biological activities. α-Asarone (1) showed remarkable hypolipidemic activity,14,15 neuroprotective effect,16 antimicrobial and insecticidal activities.17 β-Asarone (2) has been found to improve cognitive function,18 inhibit adipogenesis19 and exhibit positive effects on epilepsy.20 Asarone tablets (α-asarone, 1) have been clinically used as a bronchial asthma and bronchitis prescription drug in China. Unfortunately, toxic and genotoxic studies of α- and β-asarone (1 and 2) have indicated that these compounds may pose a risk to human health, including embryotoxicity and maternal toxicity in rats, hepatotoxicity in rat-cultivated hepatocytes, and in vivo and in vitro genotoxic damage in mammalian cells.21 The contrasting effects of α-asarone (1), i.e., its efficient therapeutic potential and toxicity, prompted us to identify analogues that exhibit a potent pharmacological effect but with low toxicity. To date, 15 asarone analogues (3–17) (Fig. 1), through hydroxylation, carbonylation and epoxidation in the C3 segment of asarone, have been isolated from Acorus L.22–27 Among these analogues, γ-asarone (3) has been isolated and identified as an isomer of α- and β-asarone (1 and 2); however, detailed biological studies have not been performed on this compound.22 (Z)-3-(2,4,5-Trimethoxyphenyl)acrylaldehyde (4), 1-(2,4,5-trimethoxyphenyl)propan-2-one (6), 1-(2,4,5-trimethoxyphenyl)propan-1-one (7) and 1-(2,4,5-trimethoxyphenyl)propan-1,2-dione (9) exhibited weak activity or induce an increase in cAMP levels at concentrations of 50 μM (P < 0.05) in N1E-115 neuroblastoma cells.23 Therefore, future studies to investigate the pharmacological toxicity and properties of compounds that are structurally analogous to asarones are necessary.
In addition to the asarone analogues, methyl eugenol derivatives are also common types of phenylpropanoids in Acorus L., including (E)-methyl isoeugenol (18),27 (Z)-methyl isoeugenol (19),27 methyl eugenol (20),13 and methyl eugenol analogues (21–24).23–25 Methyl eugenol (20) is a common component of spices and is directly added to food as a flavouring substance.28
Additional phenylpropanoids: 3′,4′,5′-trimethoxycinnamyl alcohol (25),25 1-(3,4,5-trimethoxyphenyl)-2-propene (26),13 3-(3,4,5-trimethoxyphenyl)propan-1-ol (27),24 caffeic acid (28),29 ferulic acid (29),29 (Z)-coniferyl alcohol (30)24 and trans-24-feruloyloxy-tetracosanoic acid (31)30 were also obtained (Fig. 1). Among these phenylpropanoids, (Z)-coniferyl alcohol (30) significantly inhibited nitric oxide (NO) levels (IC50 = 14.08 μM) in lipopolysaccharide (LPS)-stimulated BV-2 cells. Caffeic acid (28) tablets are used to treat or prevent bleeding during surgery, obstetrics and gynaecology, or general medicine (facilitating haemostasis). Ferulic acid (29) has been proposed as a potential treatment for numerous chronic diseases, such as Alzheimer's disease, cancer, and cardiovascular diseases, among others; however, the clinical efficacy of ferulic acid (29) requires additional documentation.31
2. Lignans
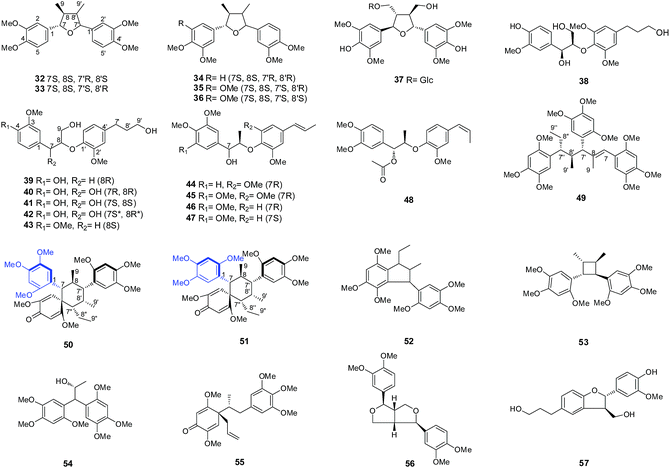
Two 7,7′-monoepoxy lignans, (+)-veraguensin (32) and galgravin (34) (Fig. 2),32 have been isolated from A. tatarinowii. Kim et al.11 have investigated the bioactive lignans from the rhizomes of A. gramineus. Ganschisandrin (33), ligraminol A (35), 5-methoxygalbelgin (36) and ligraminol B (37) (Fig. 2), were evaluated for inhibition of NO production in an activated murine microglial cell line. Compounds 35 and 37 moderately inhibited NO production, with IC50 values of 21.51 and 22.96 μM, respectively.Eleven 8-O-4′-neolignans have been identified from A. gramineus: (1R,2S)-rel-1-(4′-hydroxy-3′-methoxyphenyl)-2-[4′′-(3-hydroxypropyl)-2′′,6′′-dimethoxyphenoxy]-1,3-propanediol (38), ligraminol E (39), (7R,7R)-4,7,9,9′-tetrahydroxy-3,3′-dimethoxy-8-O-4′-neolignan (40), (7S,8S)-4,7,9,9′-tetrahydroxy-3,3′-dimethoxy-8-O-4′-neolignan (41), 7S,8R-erythro-4,7,9,9′-tetrahydroxy-3,3′-dimethoxy-8-O-4′-neolignanl (42), ligraminol D (43), (−)-(7R,8R)-virolin (44), (7R,8R)-polysyphorin (45), surinamensinols A (46) and B (47), and ligraminol C (48) (Fig. 2).11,25 Because the MeOH extract of A. gramineus rhizomes exhibited significant anti-inflammatory and cytotoxic activities, Kim et al.11,25 have investigated the anti-inflammatory effects and cytotoxic activities of these 8-O-4′-neolignans. Ligraminol D (43) and surinamensinols A (46) and B (47) significantly inhibited NO levels in LPS-stimulated BV-2 cells, with IC50 values of 18.41, 17.91 and 8.17 μM, respectively. Surinamensinols A (46) and B (47) exhibited moderate anti-proliferative activities against the A549, SK-OV-3, SK-MEL-2, and HCT-15 cell lines, with IC50 values ranging from 4.17 to 26.18 μM. In particular, surinamensinols A (46) and B (47) exhibited potent cytotoxicity against the A549 cell line, with IC50 values of 4.17 and 5.41 μM, respectively. Additionally, ligraminol D (43) exhibited weak inhibitory activity against the proliferation of the evaluated cell lines, with an IC50 value of 9.54 μM.
The tatanans (49–51) are members of novel sesquinlignans with the unprecedented carbon skeleton that is characterised by a unique C8–C7′ linkage pattern. Structurally, tatanans B and C (50 and 51) are atropisomers with a hindered rotation around the C-1–C-7 bond and possess an unprecedented spiro[5.5]undecane skeleton with two benzyl moieties attached to C-7 and C-7′ (Fig. 2, the position of hindered rotation was highlighted by blue colour).33 The possible biosynthesis pathway of the tatanans from A. tatarinowii may derive from three α- or β-asarone (1 or 2) through a complex enzymatic reaction. Tatanans A, B and C (49–51) (Fig. 2) exhibited potent and selective in vitro glucokinase-activating activities but were inactive against dipeptidyl-peptidase-4, α-glycosidase, the Na+-glucose cotransporter, and aldose reductase.33 Recently, Xiao et al.34 have reported the total synthesis of tatanan A (49) in 13 steps utilising a series of sequential [3,3]-sigmatropic rearrangements in addition to a concise enantioselective total synthesis of the more complex atropisomeric tatanans B and C (50 and 51). In contrast to the previous report, these authors utilised pure recombinant human glucokinase and demonstrated that tatanans do not function as allosteric activators of glucokinase. Therefore, future in vitro and in vivo studies are necessary to confirm these conflicting results.
In addition to these three types of lignans, additional lignan types, such as compounds 52–57,23–25,32,35,36 have also been reported (Fig. 2). Magnosalin (53) has been reported to inhibit NO levels in LPS-stimulated BV-2 cells, with an IC50 value of 18.73 μM.24
3. Sesquiterpenoids
Acorus plants are a rich source of various sesquiterpenoids, which are represented by the acoranes. Acorane-type sesquiterpenoids with the characteristic spiro[4.5]decane skeleton are biosynthesised from cis, trans-farnesyl pyrophosphate via the bisabolane cation.37 Since the isolation of acorone (58) and isoacorone (59) from the essential oil of A. calamus. in 1948,38 18 acorane derivatives (58–75)26,39–41 have been isolated from the Acorus species (Fig. 3). Pharmacological investigation of acoranes has indicated that 1-hydroxyepiacorone (61) exhibited potent anti-germination activity.26 In vitro assays have demonstrated that calamusin D (62) (10 μM) exhibited weak hepatoprotective activities against N-acetyl-p-aminophenol-induced HepG2 cell damage. Moreover, (−)-acorenone (63) exhibited weak gamma-aminobutyric acid type A receptors (GABAA) modulating properties (241% ± 23.1%; EC50 = 34.0 ± 6.7 μM).An increasing number of studies have reported the isolation and structure elucidation of sesquiterpenoids. Cadinanes represent a second major type of sesquiterpenoids from the Acorus species. Tatarinowins A (76), B (77) and C (78) have been isolated from A. tatarinowii.23,41,42 Chemical investigation of the rhizomes of A. calamus has led to the isolation of 10 cadinanes: 1,4a,6(2H)-naphthalenetriol (79),30 1,6,8a(1H)-naphthalenetriol (80),30 1,7-naphthalenediol (81),30 benghalensitriol (82),30 calamusin G (83),40 calamusin H (84),40 calamendiol (85),30 isocalamenediol (86),26 (−)-cadala-1,4,9-triene (87)43 and acorafuran (88) (Fig. 3).44
Four types of sesquiterpenoids rearranged from the germacryl cation, i.e., germacranes (89 and 90),40,45 elemanes (91–93),40,46 eudesmanes (92–98),26,30,47 and guaianes (99–105) (Fig. 3),30,40–42,48 have been identified. Moreover, studies have also identified 106–111 (Fig. 3).49
4. Diterpenoids and triterpenoids
Chemical investigation of A. tatarinowii has led to the isolation of the isopimarane diterpenes (tatarol (112) and its glycoside, tataroside (113) (Fig. 3)).50 Two triterpenoid saponins, i.e., 1β,2α,3β,19α-tetrahydroxyurs-12-en-28-oicacid-28-O-{-β-D-glucopyranosyl(1→2)}-β-D-galactopyranoside (114) and 3β,22α,24,29-tetrahydroxyolean-12-en-3-O-{-β-D-arabinosyl(1→3)}-β-D-arabinopyranoside (115) (Fig. 3), have been isolated from the extract of A. calamus.515. Amides and alkaloids
In 1997, Wang et al.52 were the first to isolate two amides from A. tatarinowii, tataramide A (116) and tataramide B (117). Additional studies on the alkaloid constituents of this plant led to the isolation of five alkaloids, acortatarin A (118), acortatarin B (119), tatarinine A (120), tatarine A (121) and 1H-pyrrole-1-butanoic acid (122).42,53 Among these alkaloids, acortatarins A–B (118 and 119) are two novel spiroalkaloids with an unusual morpholine motif. The most interesting of these compounds is acortatarin A (118), which significantly inhibited reactive oxygen species production in high glucose-stimulated mesangial cells in a dose- and time-dependent manner. Furthermore, acortatarin A (118) inhibited the high glucose-induced extracellular matrix production via the inhibition of NADPH oxidase activation, suggesting that acortatarin A (118) represents a new therapeutic candidate for diabetic nephropathy.54 For future biological studies of acortatarins, Sudhakar and co-workers have developed a synthetic strategy using readily available D-sugars as the starting material. This convergent total synthesis has revealed the revision of the absolute configuration of acortatarin A (118) and the structural revision of acortatarin B (119) (see Fig. 4).556. Miscellanous
Selcuk et al.56 were the first to investigate the constituents of the leaves of A. calamus and identified apigenin 7-O-β-D-glucoside (123) and apigenin (124). An additional eight flavonoids (125–132) have been identified from the rhizomes of the Acorus species,25,57–62 including two flavonol glycosides (126, 129)57,58 and a flavone C-glycoside (130).60 Apigenin (124), which is abundantly present in common fruits and vegetables, has been demonstrated to possess substantial anti-oxidant, anti-inflammatory and anti-carcinogenic properties.63 A clinical trial to verify the hypothesis that dietary supplementation with bioflavonoids will diminish the recurrence rate of colonic neoplasia will begin in May 2015.64Lee et al.65 have identified three new quinone derivatives (133–135) from A. gramineus, which exhibited significant anti-inflammatory effects via the reduction of NO levels in LPS-stimulated BV-2 cells.
Eleven sterols (136–146) have been identified in Acorus L., most of which are stigmasterol, daucosterol and sitosterol derivatives.30,32,66 Rai et al.67 have isolated and characterised a xanthone glycoside (147) from the rhizomes of A. calamus. Yang et al.68 have investigated the decoction of the rhizomes of A. tatarinowii. Four compounds were identified as 2,5-dimethoxybenzoquinone (148), benzoic acid (149) and furfuraldehydes (150 and 151). Detailed information on these compounds is summarised in Table 1.
| NO. | Chemical name | Part | Source plant | Ref. |
|---|---|---|---|---|
| 123 | Apigenin 7-O-β-D-glucoside | Leaves | A. calamus | 56 |
| 124 | Apigenin | Leaves | A. calamus | 56 |
| 125 | Norizalpinin | Rhizomes | A. calamus | 57 |
| 126 | Galangin-3-O-β-D-glucopyranosyl-7-O-β-L-rhamnopyranoside | Roots | A. calamus | 58 |
| 127 | 3-O-Methylkaempferol | Rhizomes | A. gramineus | 25 |
| 128 | Noranhydroicaritin | Rhizomes | A. tatarinowii | 59 |
| 129 | Luteolin-8-C-β-D-glucopyranoside | Roots | A. calamus | 60 |
| 130 | Luteolin 6,8 C-diglucoside | — | A. calamus | 61 |
| 131 | 5,4′-Dihydroxy-7,8-dimethoxyflavone | Rhizomes | A. calamus | 62 |
| 132 | 5-Hydroxy-7,8,3′,4′-tetramethoxyflavone | Rhizomes | A. calamus | 63 |
| 133 | (7′R,8R,8′S)-7′-(2′,4′,5′-Trimethoxyphenyl)-4,7α,8-trimethoxy-8,8′-dimethyl-2,5-quinone | Rhizomes | A. tatarinowii | 65 |
| 134 | 7′-(2′,4′,5′-Trimethoxyphenyl)-4-methoxy-8,8′-dimethyl-2,5-quinone | Rhizomes | A. tatarinowii | 65 |
| 135 | 1-cis-Propenyl-1S,6R-epoxy-4-methoxy-2,5-quinone | Rhizomes | A. tatarinowii | 65 |
| 136 | β-Stigmasterol | Rhizomes | A. tatarinowii | 32 |
| 137 | β-Sitosterol | Rhizomes | A. calamus | 66 |
| 138 | 7β-Hydroxy-β-sitosterol | Rhizomes | A. calamus | 66 |
| 139 | 7α-Hydroxy-β-sitosterol | Rhizomes | A. calamus | 66 |
| 140 | Daucosterin | Rhizomes | A. calamus | 66 |
| 141 | Stigmasta-5,25-diene | Rhizomes | A. calamus | 30 |
| 142 | 4′-O-Docosanoyl-3-O-β-D-glucosyl-sitosterol | Rhizomes | A. calamus | 66 |
| 143 | Stigmast-4-ene-6β-ol-3-one | Rhizomes | A. calamus | 66 |
| 144 | 6β-Hydroxystigmasta-4,22-diene-3-one | Rhizomes | A. calamus | 66 |
| 145 | Stigmast-5-en-3β-ol-7-one | Rhizomes | A. calamus | 30 |
| 146 | Stigmasta-5,22-dien-3β-ol-7-one | Rhizomes | A. calamus | 30 |
| 147 | 4,5,8-Trimethoxy-xanthone-2-O-β-D-glucopyranosyl(1→2)-O-β-D-galactopyranoside | Rhizomes | A. calamus | 67 |
| 148 | 2,5-Dimethoxybenzoquinone | Rhizomes | A. tatarinowii | 68 |
| 149 | 4-Hydroxy-3-methoxybenzoic acid | Rhizomes | A. tatarinowii | 68 |
| 150 | 5-Hydroxymethyl-2-furaldehyde | Rhizomes | A. tatarinowii | 68 |
| 151 | 2-Furancarboxaldehyde-5,5′[oxybis (methylene)] | Rhizomes | A. tatarinowii | 68 |
Neuropharmacology
The Acorus species exhibits significant CNS actions, such as anticonvulsant, neuroprotective, memory enhancing and sedative properties, which validates its use to treat certain CNS diseases in the ayurvedic, Chinese and other medicinal systems (Table 2).| Traditional use | Pharmacological activity | Planta | Parts/constituent | Assay/study | Result/activity | Ref. |
|---|---|---|---|---|---|---|
| a AT (A. tatarinowii); AC (A. calamus); AG (A. gramineus). | ||||||
| Epilepsy | Anticonvulsant antiepileptic | AT | Decocting extract | PTZ kindling model | Prevent convulsion-related GABAergic neuron damage | 10 |
| Volatile oil | PTZ kindling model | Less effective | ||||
| AC | Rhizomes | Maximal electro shock seizure model | Decreased the duration of tonic extensor phase | 69 | ||
| AC | Rhizomes | FeCl3-induced epileptogenesis | Decreased | 70 | ||
| α-Asarone (1) | Induced inward currents | EC50 = 248 ± 33 μM | 71 | |||
| PTZ or kainate injection model | Prolonged the latency to clonic and tonic seizures | |||||
| Reduced the mortality | ||||||
| α-Asarone (1) | Mice and rats seizure models | Effective anticonvulsant activity | 72 and 73 | |||
| α-Asarone (1) | Epileptic patients | Effective rate: 59% | 74 | |||
| Memory disorders | Neuroprotection | AC | Ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1 water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) extract 1) extract |
Increased in lipid peroxidation | Cortex, 157%; corpus striatum, 58% | 75 |
| Decreased in glutathione levels | Cortex, 59%; corpus striatum, 34% | |||||
| Decreased superoxide dismutase (SOD) activity | Cortex, 64%; corpus striatum, 32% | |||||
| Rota-rod performance and grid walking test | Significant improvement in neurobehavioural performance | |||||
| AG | Essential oil | Inhibited glutamate-induced excitotoxicity | IC50 = 0.241 mg mL−1 | 76 | ||
| Inhibited toxicity induced by NMDA | IC50 = 0.139 mg mL−1 | |||||
| AG | Rhizomes | Cytotoxic action of Aβ (1–40) | Decreased Aβ (1–40)-induced cell death | 77 | ||
| β-Asarone (2) | Basal Ca2+ intake | Weak inhibited | ||||
| Eugenol | Aβ-induced Ca2+ intake | 10 μM (50% response) | ||||
| α-Asarone (1) | Pentylenet etrazol induced epileptic seizures in immature rats | Reduced the number of apoptotic hippocampal neurons | 78 | |||
| Cognitive decline, amnesia, dementia, memory disorders | Memory enhancement | AG | Methanol extract | Ischemia-induced cell death | Reduced cell death in the hippocampal CA1 area | 79 |
| Morris water maze test | Significant improvement in escape latency to find the platform | |||||
| Radial eight-arm maze test | Improvement of the number of choice errors | |||||
| AG | Essential oil | Step-down passive avoidance test and Y maze | Improved the latency and number of errors | 80 | ||
| Levels of norepinephrine, dopamine and serotonin | Increased | |||||
| Levels of acetylcholinesterase | Decreased | |||||
| β-Asarone (2) | Aβ hippocampus injection rats | Ameliorated Aβ (1–42)-induced cognitive impairment and reversed the increase of apoptosis in the hippocampus | 18 | |||
| AC | Methanol extract | In vitro AChE assay | IC50 = 200 mg mL−1 | 81 | ||
| AC | Ethanol extract | In vitro AChE assay | IC50 = 182.31 ± 16.78 mg mL−1 | 82 | ||
| Essential oil | IC50 = 10.67 ± 0.81 mg mL−1 | |||||
| β-Asarone (2) | IC50 = 3.33 ± 0.02 μM | |||||
| α-Asarone (1) | IC50 = 46.38 ± 2.69 μM | |||||
| Sedation, analgesia, schizophrenia | Sedative | AC | Ethanol extract | Norepinephrine level | Cerebral cortex, increased; midbrain and cerebellum, decreased | 83 |
| Serotonin level | Cerebral cortex, increased; midbrain, decreased | |||||
| Dopamine level | Caudate nucleus and midbrain, increased; cerebellum, decreased | |||||
| β-Asarone (2) | Locomotor analysis | Significant decrease in the total number of crossings | 7 | |||
| Body temperature | Hypothermic | |||||
| [3H]-CP 55940 binding assay | Exert a direct agonistic activity on CB1 receptors | |||||
1. Anticonvulsant
The anticonvulsant activity of Acorus extracts have been studied in vivo, validating the traditional use of this herb as an anticonvulsant and antiepileptic. The decoction and volatile oil from the rhizomes of A. tatarinowii were extracted by traditional decocting and supercritical CO2 fluid extraction methods. Both the decoction extract and the volatile oil can prevent convulsions and convulsion-related GABAergic neuronal damage in the brain in the prolonged pentylenetetrazol (PTZ) kindling model. The volatile oil exhibited less efficacy for PTZ-induced convulsions.10 To compare the anticonvulsant activity, the raw and classically processed rhizomes of A. calamus were screened against the maximal electroshock seizure model to evaluate the influence of the classical purification procedure on the pharmacological action of A. calamus. The raw and classically processed samples exhibited significant anticonvulsant activity by decreasing the duration of the tonic extensor phase.69 A. calamus has also been demonstrated to possess the ability to prevent the development of FeCl3-induced epileptogenesis by modulating antioxidant enzymes; this finding suggests the potential of A. calamus for development as an effective antiepileptic drug.70 Huang et al.71 have characterised the action of α-asarone (1) on the excitability of rat hippocampal neurons in culture and on the epileptic activity induced by PTZ or kainate injection in vivo. Under the whole-cell configuration, α-asarone (1) induced inward currents in a dose-dependent manner, with an EC50 value of 248 ± 33 μM. These results suggested that α-asarone (1) inhibited the activity of hippocampal neurons and produced an antiepileptic effect in the CNS by enhancing tonic GABAergic inhibition. Additional studies on α-asarone (1) in various animal seizure models have suggested that α-asarone (1) exhibits a favourable antiepileptic activity.72,73 In a clinical trial, Pan et al.74 found that α-asarone (1) (30–90 mg, p.o., tid) to be effective in 59% of 32 episodes of grand mal epilepsy. And in the control group, 8 of 15 epileptic patients were controlled by treating with phenytoin (0.1 g, p.o., bid-tid). There was no significant difference between these two groups. Importantly, the advantage of fewer adverse effects and larger safety dosage range suggested that α-asarone (1) may be the drug of choice for these particular grand mal epilepsy patients.2. Neuroprotection
A potential neuroprotective activity of the ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) extract of the rhizomes of A. calamus has been reported using a middle cerebral artery occlusion-induced ischaemia model. Ischaemic rats treated with A. calamus exhibited significant improvement in neurobehavioural performance, increased reduced glutathione levels and SOD activity in both the cortex and the corpus striatum, and an improved neurological function score.75 In the study evaluating the effects of the essential oil (EO) from A. gramineus, EO inhibited the glutamate-induced excitotoxicity in a dose-dependent manner, with an IC50 value of 0.241 mg mL−1. EO exerted a more potent neuroprotection against the toxicity induced by NMDA (IC50 = 0.139 mg mL−1). Receptor-ligand binding studies have revealed that EO dramatically inhibited the specific binding of a use-dependent NMDA receptor-ion channel blocker [3H]MK-801, indicating an NMDA receptor antagonist-like action.76 The effects of the water extracts of six medicinal herbs on the cytotoxic action of amyloid-β1-40 (Aβ1-40) have been evaluated in PC-12 cells, and only the A. gramineus extract significantly decreased Aβ1-40-induced cell death. Furthermore, eugenol and β-asarone (2) were isolated and identified as the major active constituents. Purified eugenol and β-asarone (2) protected PC-12 cells from the toxic effect of Aβ1-40.77 A randomised controlled animal study has indicated that A. gramineus and α-asarone (1) increased Bcl-2 expression, decreased Bax expression, and reduced the number of apoptotic hippocampal neurons during PTZ-induced epileptic seizures in immature rats.78
1) extract of the rhizomes of A. calamus has been reported using a middle cerebral artery occlusion-induced ischaemia model. Ischaemic rats treated with A. calamus exhibited significant improvement in neurobehavioural performance, increased reduced glutathione levels and SOD activity in both the cortex and the corpus striatum, and an improved neurological function score.75 In the study evaluating the effects of the essential oil (EO) from A. gramineus, EO inhibited the glutamate-induced excitotoxicity in a dose-dependent manner, with an IC50 value of 0.241 mg mL−1. EO exerted a more potent neuroprotection against the toxicity induced by NMDA (IC50 = 0.139 mg mL−1). Receptor-ligand binding studies have revealed that EO dramatically inhibited the specific binding of a use-dependent NMDA receptor-ion channel blocker [3H]MK-801, indicating an NMDA receptor antagonist-like action.76 The effects of the water extracts of six medicinal herbs on the cytotoxic action of amyloid-β1-40 (Aβ1-40) have been evaluated in PC-12 cells, and only the A. gramineus extract significantly decreased Aβ1-40-induced cell death. Furthermore, eugenol and β-asarone (2) were isolated and identified as the major active constituents. Purified eugenol and β-asarone (2) protected PC-12 cells from the toxic effect of Aβ1-40.77 A randomised controlled animal study has indicated that A. gramineus and α-asarone (1) increased Bcl-2 expression, decreased Bax expression, and reduced the number of apoptotic hippocampal neurons during PTZ-induced epileptic seizures in immature rats.78
3. Memory enhancement
To investigate whether A. gramineus (AG) influenced cerebral ischaemia-induced neuronal and cognitive impairments, Lee et al.79 have examined the effect of AG on ischaemia-induced cell death in the striatum, cortex and hippocampus and on learning and memory-impaired rats in the Morris water maze and radial eight-arm maze. AG exhibited a protective effect against ischaemia-induced neuronal loss and learning and memory damage. Geng et al.18 have investigated the effects of β-asarone (2) on cognitive function and neuronal apoptosis in rats subjected to Aβ injection in the hippocampus and have studied its mechanism of action. Oral administration of β-asarone (2) (12.5, 25, or 50 mg kg−1 for 28 days) ameliorated the Aβ (1–42)-induced cognitive impairment and reversed the increase in apoptosis in the hippocampus. β-Asarone (2) attenuated the Aβ (1–42)-induced neuronal apoptosis in the hippocampus via reversal of the down-regulation of Bcl-2 and Bcl-w, caspase 3 activation, and c-Jun N-terminal kinase phosphorylation. Moreover, the essential oil extracted from the rhizomes of AG improved cognitive function in aged animals, possibly by increasing the relative levels of norepinephrine, dopamine and serotonin and by decreasing the activity of acetylcholinesterase (AChE) in the cerebra.80 Because cognitive performance and memory are related to acetylcholine levels, several studies have illustrated that the AChE inhibitory effect of the genus Acorus may account for its traditional use. The methanol extract of A. calamus exhibited significant AChE inhibition at a concentration of 200 mg mL−1.81 The in vitro AChE inhibitory effect of the ethanol extract, the essential oil of the rhizomes of A. calamus, and its major constituents have been evaluated using Ellman's method. The IC50 values obtained for the ethanol extract, the essential oil, β-asarone (2) and α-asarone (1) were 182.31 ± 16.78 μg mL−1, 10.67 ± 0.81 μg mL−1, 3.33 ± 0.02 μM and 46.38 ± 2.69 μM, respectively.824. Sedative
The ethanol extract of A. calamus exerts its depressive action by altering the electrical activity and differentially altering monoamine levels in different regions of the brain.83 Zanoli et al.7 have found that β-asarone (2) exerted sedative and hypothermic, but not analgesic effects. When administered with the cannabinomimetic drug WIN 55-212-2, β-asarone (2) potentiated certain typical behavioural activities induced by cannabinoids in animals. Binding assays, which were performed on cortical synaptic membrane preparations using a specific cannabinoid radioligand ([3H]CP-55, 940), indicated that β-asarone (2) does not exert a direct agonistic activity on CB1 receptors. Therefore, β-asarone (2) cannot be considered a pure cannabinomimetic agent, although it may act as an allosteric modulatory agent.Conclusion
As reviewed herein, chemical investigation of the Acorus species has revealed rich secondary metabolites, whereas only asarones have demonstrated significant effects on different biological properties, particularly anticonvulsant, neuroprotective, and memory enhancing effects. Future studies are necessary to identify additional constituents that exhibit potent pharmacological effects at low doses. Nevertheless, the differences in the chemical compositions among the Acorus species remain unknown, which presents challenges in validating their different traditional uses. The elucidation of the chemical compositions of Acorus species will expand the medicinal resources of Acorus and will significantly protect their native plant resources.Traditional uses of the Acorus species were, in most cases, supported by pharmacological studies, particularly for the treatment of CNS diseases. Although beneficial effects on the treatment of convulsive and epileptic diseases have been demonstrated in in vitro and in vivo models, more clinical trials still be required. The potential for neuroprotective and cognitive and memory improvement activities has suggested that Acorus represents a promising treatment for dementia or other diseases with cognitive decline, such as Alzheimer's disease. To properly evaluate the results of these studies, the Acorus species are assumed to act as a ‘delivering servant’ or with a ‘Kaiqiao’ effect in TCM formulas for the treatment of CNS diseases and are capable of increasing the uptake of active compounds in the brain.84,85 Although studies have suggested that Acorus can increase the permeability of the blood–brain barrier86 and facilitate the uptake of ginsenosides Rg1, Re and Rb1 in the brain following oral administration of Kai-Xin-San preparations,87 additional studies are necessary to elucidate their precise mechanism in the treatment of CNS diseases. Despite the important and varied phytochemical and neuropharmacological studies available, clinical trials are necessary to confirm the use of this species in medical practice.
Acknowledgements
This work was financially supported by grants from the National Natural Science Foundation of China (81422054, 81172945), the Fundamental Research Funds for the Central Universities (21614301), the Guangdong Natural Science Funds for Distinguished Young Scholar (S2013050014287), and the Programme of Introducing Talents of Discipline to Universities (B13038).Notes and references
- The Angiosperm phylogeny Group, Ann. Mo. Bot. Gard., 1998, 85, 531–553 CrossRef.
- The Angiosperm phylogeny Group, Bot. J. Linn. Soc., 2009, 161, 105–121 CrossRef PubMed.
- Flora of China Editorial Committee, Acoraceae through Cyperaceae, Flora of China, Science Press, Beijing, 2010, pp. 1–2 Search PubMed.
- Y. Y. Chen, D. Z. Li and H. Wang, Acta Bot. Yunnanica, 2002, 24, 699–706 CAS.
- H. Li, Araceae, Flora Repubulicae Popularis Sinicae, Science Press, Beijing, 1979, vol. 13, ch. 2, pp. 7–8 Search PubMed.
- H. Gilani Anwar Ul, J. Shah Abdul, M. Ahmad and F. Shaheen, Phytother. Res., 2006, 20, 1080–1084 CrossRef PubMed.
- P. Zanoli, R. Avallone and M. Baraldi, Phytother. Res., 1998, 12, 114–116 CrossRef.
- P. K. Mukherjee, V. Kumar, M. Mal and P. J. Houghton, Pharm. Biol., 2007, 45, 651–666 CrossRef CAS.
- K. R. Kirtikar and B. D. Basu, Indian Medicinal Plants, M/s Bishen Sing, Mahendra Pal Sing Publishers, Dehradun, 1987, vol. IV, pp. 1229–1230 Search PubMed.
- W. P. Liao, L. Chen, Y. H. Yi, W. W. Sun, M. M. Gao, S. Tao and S. Q. Yang, Epilepsia, 2005, 46, 21–24 CrossRef PubMed.
- K. H. Kim, H. K. Kim, S. U. Choi, E. Moon, S. Y. Kim and K. R. Lee, J. Nat. Prod., 2011, 74, 2187–2192 CrossRef CAS PubMed.
- X. Y. Yang and F. K. Chen, Shenyang Yaoke Daxue Xuebao, 1999, 16, 71–78 CAS.
- G. Mazza, J. Chromatogr., 1985, 328, 179–194 CrossRef CAS.
- C. Gomez, G. Chamorro, M. A. Chavez, G. Martinez, M. Salazar and N. Pages, Plant. Med. Phytother., 1987, 21, 279–284 CAS.
- L. Rodríguez-Páez, M. Juárez-Sanchez, J. Antúnez-Solís, I. Baeza and C. Wong, Phytomedicine, 2003, 10, 397–404 CrossRef.
- I. D. Limón, L. Mendieta, A. Díaz, G. Chamorro, B. Espinosa, E. Zenteno and J. Guevara, Neurosci. Lett., 2009, 453, 98–103 CrossRef PubMed.
- R. A. Momin and M. G. Nair, J. Agric. Food Chem., 2002, 50, 4475–4478 CrossRef CAS PubMed.
- Y. T. Geng, C. C. Li, J. C. Liu, G. H. Xing, L. Zhou, M. X. Dong, X. Y. Li and Y. C. Niu, Biol. Pharm. Bull., 2010, 33, 836–843 CAS.
- M. H. Lee, Y. Y. Chen, J. W. Tsai, S. C. Wang, T. Watanabe and Y. C. Tsai, Food Chem., 2011, 126, 1–7 CrossRef CAS PubMed.
- Y. Q. Fang, R. M. Fang, G. L. Fang, Y. Jiang and S. Y. Fu, Zhongguo Zhongyao Zazhi, 2008, 33, 534–536 CAS.
- M. Cassani-Galindo, E. Madrigal-Bujaidar, G. Chamorro, F. Díaz, J. Tamariz and J. J. Espinosa-Aguirre, Toxicol. in Vitro, 2005, 19, 547–552 CrossRef CAS PubMed.
- A. K. Sinha, R. Acharya and B. P. Joshi, J. Nat. Prod., 2002, 65, 764–765 CrossRef CAS PubMed.
- X. G. Tong, G. S. Wu, C. G. Huang, Q. Lu, Y. H. Wang, C. L. Long, H. R. Luo, H. J. Zhu and Y. X. Cheng, J. Nat. Prod., 2010, 73, 1160–1163 CrossRef CAS PubMed.
- K. H. Kim, E. Moon, H. K. Kim, J. Y. Oh, S. Y. Kim, S. U. Choi and K. R. Lee, Bioorg. Med. Chem. Lett., 2012, 22, 6155–6159 CrossRef CAS PubMed.
- C. H. Park, K. H. Kim, I. K. Lee, S. Y. Lee, S. U. Choi, J. H. Lee and K. R. Lee, Arch. Pharmacal Res., 2011, 34, 1289–1296 CrossRef CAS PubMed.
- K. Nawamaki and M. Kuroyanagi, Phytochemistry, 1996, 43, 1175–1182 CrossRef CAS.
- M. Della Greca, P. Monaco, L. Previtera, G. Aliotta, G. Pinto and A. Pollio, Phytochemistry, 1989, 28, 2319–2321 CrossRef CAS.
- R. L. Smith, T. B. Adams, J. Doull, V. J. Feron, J. I. Goodman, L. J. Marnett, P. S. Portoghese, W. J. Waddell, B. M. Wagner, A. E. Rogers, J. Caldwell and I. G. Sipes, Food Chem. Toxicol., 2002, 40, 851–870 CrossRef CAS.
- Y. Dong, R. B. Shi and B. Liu, Zhongguo Yaoye, 2008, 17, 18–19 CAS.
- W. W. Dong, D. J. Yang and R. H. Lu, Planta Med., 2010, 76, 454–457 CrossRef CAS PubMed.
- C. Mancuso and R. Santangelo, Food Chem. Toxicol., 2014, 65, 185–195 CrossRef CAS PubMed.
- Y. Dong, R. B. Shi and B. Liu, Beijing Zhongyiyao Daxue Xuebao, 2007, 30, 61–63 CAS.
- G. Ni, Z. F. Shen, Y. Lu, Y. H. Wang, Y. B. Tang, R. Y. Chen, Z. Y. Hao and D. Q. Yu, J. Org. Chem., 2011, 76, 2056–2061 CrossRef CAS PubMed.
- Q. Xiao, J. J. Jackson, J. M. Bowler, A. Basak, B. G. Miller and A. Zakarian, Nat. Chem., 2013, 5, 410–416 CrossRef CAS PubMed.
- D. B. Saxena, Phytochemistry, 1986, 25, 553–555 CrossRef CAS.
- T. D. Nguyen, T. H. Nguyen, D. T. Pham and W. C. Taylor, Tap Chi Hoa Hoc, 2007, 45, 356–362 CAS.
- Y. Hashidoko, S. Tahara and J. Mizutani, Phytochemistry, 1991, 30, 3729–3739 CrossRef CAS.
- F. Sorm, V. Herout and V. Terpenes, Collect. Czech. Chem. Commun., 1948, 13, 177–205 CrossRef CAS.
- J. Vrkoc, V. Herout and F. Sorm, Collect. Czech. Chem. Commun., 1961, 26, 3183–3185 CrossRef CAS.
- Z. Y. Hao, D. Liang, H. Luo, Y. F. Liu, G. Ni, Q. J. Zhang, L. Li, Y. K. Si, H. Sun, R. Y. Chen and D. Q. Yu, J. Nat. Prod., 2012, 75, 1083–1089 CrossRef CAS PubMed.
- X. L. Feng, Y. Yu, H. Gao, Z. Q. Mu, X. R. Cheng, W. X. Zhou and X. S. Yao, RSC Adv., 2014, 4, 42071–42077 RSC.
- X. G. Tong, B. Qiu, G. F. Luo, X. F. Zhang and Y. X. Cheng, J. Asian Nat. Prod. Res., 2010, 12, 438–442 CrossRef CAS PubMed.
- M. Rohr and P. Naegeli, Phytochemistry, 1979, 18, 328–329 CrossRef CAS.
- A. V. Tkachev, A. M. Gur'ev and M. S. Yusubov, Chem. Nat. Compd., 2006, 42, 696–698 CrossRef CAS.
- M. Niwa, A. Nishiyama, M. Iguchi and S. Yamamura, Bull. Chem. Soc. Jpn., 1975, 48, 2930–2934 CrossRef CAS.
- H. Minato, R. Fujioka and K. Takeda, Chem. Pharm. Bull., 1971, 19, 638–640 CrossRef CAS.
- M. Niwa, A. Nishiyama, M. Iguchi and S. Yamamura, Chem. Lett., 1972, 9, 823–826 CrossRef.
- M. Rohr, P. Naegeli and J. J. Daly, Phytochemistry, 1979, 18, 279–281 CrossRef CAS.
- H. Ueda, C. Katayama and J. Tanaka, Bull. Chem. Soc. Jpn., 1980, 53, 1263–1266 CrossRef CAS.
- M. F. Wang, A. N. Lao and H. C. Wang, Chin. Chem. Lett., 1997, 8, 37–38 CAS.
- R. Rai, I. R. Siddiqui and J. Singh, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1998, 37, 473–476 Search PubMed.
- M. F. Wang, A. N. Lao and H. C. Wang, Chin. Chem. Lett., 1997, 8, 35–36 CrossRef CAS.
- X. G. Tong, L. L. Zhou, Y. H. Wang, C. F. Xia, Y. Wang, M. Liang, F. F. Hou and Y. X. Cheng, Org. Lett., 2010, 12, 1844–1847 CrossRef CAS PubMed.
- Z. F. Zhao, L. L. Zhou, X. Chen, Y. X. Cheng, F. F. Hou and J. Nie, Chin. Med. J., 2013, 126, 1230–1235 CAS.
- G. Sudhakar, V. D. Kadam, S. Bayya, G. Pranitha and B. Jagadeesh, Org. Lett., 2011, 13, 5452–5455 CrossRef CAS PubMed.
- S. S. Selcuk, M. Bahar and A. H. Mericli, Acta Pharm. Sci., 2009, 51, 83–85 CAS.
- A. Patra and A. K. Mitra, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1979, 17, 412–414 Search PubMed.
- V. K. Saxena and P. Saxena, J. Inst. Chem., 2005, 77, 40–43 CAS.
- H. Tao, E. Y. Zhu and Z. T. Wang, Zhongguo Tianran Yaowu, 2006, 4, 159–160 CAS.
- V. K. Saxena and P. Saxena, J. Inst. Chem., 2009, 81, 39–43 CAS.
- E. G. El'yashevich, G. A. Drozd, K. E. Koreshchuk, N. I. Bezuglaya, V. I. Manych and L. I. Shestak, Khim. Prir. Soedin., 1974, 10, 94 Search PubMed.
- C. Q. Xiao, L. J. Weng, X. Y. Zhang, X. Zhao and C. X. Zhou, Chin. Tradit. Herb. Drugs, 2008, 39, 1463–1465 CAS.
- S. Shukla and S. Gupta, Pharm. Res., 2010, 27, 962–978 CrossRef CAS PubMed.
- Clinical Trials.gov, http://clinicaltrials.gov/ct2/show/NCT00609310?term=Apigenin%26rank=1, (accessed Nov 2014).
- S. Y. Lee, E. J. Moon, S. Y. Kim, S. U. Choi and K. R. Lee, Biosci., Biotechnol., Biochem., 2013, 77, 276–280 CAS.
- W. W. Dong, W. Jiao, M. C. Deng, C. B. Yang, J. M. Yue and R. H. Lu, J. Chin. Chem. Soc., 2008, 55, 1277–1279 CAS.
- R. Rai, A. Gupta, I. R. Siddiqui and J. Singh, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1999, 38, 1143–1144 Search PubMed.
- X. Y. Yang, F. C. Chen and L. J. Wu, Chin. Tradit. Herb. Drugs, 1998, 29, 730–731 Search PubMed.
- S. D. Bhat, B. K. Ashok, R. N. Acharya and B. Ravishankar, Ayu, 2012, 33, 119–122 CrossRef PubMed.
- R. Hazra, K. Ray and D. Guha, Hum. Exp. Toxicol., 2007, 26, 947–953 CAS.
- C. Huang, W. G. Li, X. B. Zhang, L. Wang, T. L. Xu, D. Z. Wu and Y. Li, Neuropharmacology, 2013, 65, 1–11 CrossRef CAS PubMed.
- J. K. Miao, Q. X. Chen, X. M. Wu, C. Li and X. P. Zhang, Int. J. Pharmacol., 2012, 8, 567–571 CAS.
- Q. X. Chen, J. K. Miao, C. Li, X. W. Li, X. M. Wu and X. P. Zhang, Biol. Pharm. Bull., 2013, 36, 23–30 Search PubMed.
- Y. Z. Pan, J. S. Zhu and Y. D. Zhang, Xinyao Yu Linchuang, 1990, 9, 89–91 Search PubMed.
- P. K. Shukla, V. K. Khanna, M. M. Ali, R. Maurya, M. Y. Khan and R. C. Srimal, Hum. Exp. Toxicol., 2006, 25, 187–194 Search PubMed.
- J. Cho, J. Y. Kong, D. Y. Jeong, K. D. Lee, D. U. Lee and B. S. Kang, Life Sci., 2001, 68, 1567–1573 CrossRef CAS.
- Y. Irie and W. M. Keung, Brain Res., 2003, 963, 282–289 CrossRef CAS.
- L. B. Yang, S. L. Li, Y. Z. Huang, J. M. Liang and Y. H. Wang, Neural Regener. Res., 2008, 3, 19–24 CAS.
- B. Lee, Y. K. Choi, H. Kim, S. Y. Kim, D. H. Hahm, H. J. Lee and I. Shim, Life Sci., 2003, 74, 435–450 CrossRef CAS PubMed.
- H. Zhang, T. Han, C. H. Yu, K. Rahman, L. P. Qin and C. Peng, J. Pharm. Pharmacol., 2007, 59, 301–309 CrossRef CAS PubMed.
- M. H. Oh, P. J. Houghton, W. K. Whang and J. H. Cho, Phytomedicine, 2004, 11, 544–548 CrossRef CAS PubMed.
- P. K. Mukherjee, V. Kumar, M. Mal and P. J. Houghton, Planta Med., 2007, 73, 283–285 CrossRef CAS PubMed.
- R. Hazra and D. Guha, Biog. Amines, 2003, 17, 161–169 CrossRef CAS PubMed.
- L. F. Zhu and Y. Fang, Shuli Yiyaoxue Zazhi, 1998, 11, 46–47 Search PubMed.
- Z. Y. Hu, Y. Huang, G. Liu, F. Liu, W. X. Zhou and Y. X. Zhang, Zhongyao Yaoli Yu Linchuang, 2012, 28, 252–256 Search PubMed.
- Y. Hu, M. Yuang, P. Liu, L. H. Mu and H. Wang, China J. Chin. Mater. Med., 2009, 34, 349–351 Search PubMed.
- W. Wang, Q. P. Liao, L. H. Quan, C. Y. Liu, Q. Chang, X. M. Liu and Y. H. Liao, J. Ethnopharmacol., 2010, 131, 313–320 CrossRef CAS PubMed.
Footnote |
| † These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |