DOI:
10.1039/C4RA11973H
(Paper)
RSC Adv., 2015,
5, 17000-17006
Synthesis and characterization of a Sr0.95Y0.05TiO3−δ-based hydrogen electrode for reversible solid oxide cells
Received
8th October 2014
, Accepted 23rd January 2015
First published on 23rd January 2015
Abstract
Reversible solid oxide cells (RSOCs) can generate electricity as solid oxide fuel cells (SOFC) facing a shortage of electricity and can also store the electricity as solid oxide electrolysis cells (SOEC) at the time of excessive electricity. The composite Sr0.95Y0.05TiO3−δ–Sm0.2Ce0.8O1.9 (SYT–SDC) as the hydrogen electrode provides a promising alternative for a conventional Ni/YSZ. The possible charge compensation mechanism of SYT is described as Sr0.95Y0.05Ti0.95−2δ4+Ti2δ+0.053+O3−δ. The Ti3+ is approximately 11.73% in the reduced SYT by XRD Rietveld refinement, electron paramagnetic resonance (EPR) and thermogravimetry (TG) analysis. Voltage–current curves and impedance spectra are measured as a function of applied voltages to characterize the cells. The bulk resistance (Ro) and the electrode polarization resistance (Rp) at open circuit voltages (OCV) at 750 °C are 9.06 Ω cm2 and 10.57 Ω cm2, respectively. The Ro values have a small amount of changes with small slopes both in the SOFC (−0.29 Ω cm2 V−1) and SOEC mode (0.5 Ω cm2 V−1), whereas the Rp values decrease all the time with the increasing voltages at both the SOFC (−2.59 Ω cm2 V−1) and SOEC mode (−9.65 Ω cm2 V−1), indicating that the electrical conductivity and electro-catalytic property of the SYT-based hydrogen electrode can be improved under the SOEC mode.
1. Introduction
Reversible solid oxide cells (RSOCs) have drawn more and more attention as a cost-effective energy regeneration and storage system due to its promising solution to the continuous supply of electrical energy, which could play a role of solid oxide fuel cells (SOFC) at the time of electricity shortage, and could also act as solid oxide electrolysis cells (SOEC) for the use of excessive electricity.1–4 Generally, SOFC and SOEC modes essentially differ in the chemical potential gradient and electrical potential gradient, leading to electrode materials with considerably higher requirements for meeting the performance and long-time stability of RSOCs.5,6
Ni/YSZ cermet, a conventional SOFC anode material, is directly used as a hydrogen electrode material for high-temperature steam electrolysis because of its excellent catalytic properties and suitable thermal expansion behavior. However, the pre-reduction of the Ni/YSZ hydrogen electrode and a proper concentration of reducing gas are required to prevent the oxidization of metallic Ni, which can increase the polarization resistance and even cause the failure of long-time testing.7–10 Accordingly, a Ni-based electrode may be inappropriate for RSOCs based on the abovementioned challenges, and thus, it is necessary to find a nickel-free, more stable and highly active hydrogen electrode for RSOCs to obtain high performance and long-term stability. (La0.75Sr0.25)xCr0.5Mn0.5O3 (LSCM) based materials have been widely reported to be electrochemically active and redox-stable SOFC anode materials, which can be potentially used as SOEC hydrogen electrode materials.11–13 However, under strong reducing potential conditions or high voltage applied to electrolysis, unfortunately, LSCM would be unstable with structural transformation.14,15 The perovskites, SrTiO3 with doping donors such as La3+ or Y3+ on the Sr2+ sites, are active and redox-stable materials that have high n-type conductivity upon reduction and are highly attractive as electrode materials.16–25 Kim et al.24 reported that the electrochemical reaction kinetic and the number of active electrochemical reaction sites of Sr0.92Y0.08TiO3−δ anode by Sm0.2Ce0.8O1.9 (SDC) coating were greatly improved. To the best of our knowledge, Sr0.95Y0.05TiO3−δ–Sm0.2Ce0.8O1.9 (SYT–SDC) as the hydrogen electrode has not been reported to date. In this study, the oxidized and reduced SYT samples have been investigated by XRD Rietveld refinement, electron paramagnetic resonance (EPR) and thermogravimetry (TG) analysis. Voltage–current curves and impedance spectra with the RSOCs configuration of (hydrogen electrode) SYT–SDC/YSZ/LSM–YSZ (air electrode) were measured as a function of applied voltages.
2. Experimental
2.1 Preparation of SYT, SDC, YSZ and LSM powders
The SYT powder was prepared through an EDTA–citrate complexation process. A suitable amount of EDTA was dissolved into distilled water with ammonia added to obtain a transparent solution. First, Ti(OCH(CH3)2)4 was dissolved into a nitrate solution, which was then mixed with the nitrate solution of Sr2+ and Y3+ in the designed ratio and was blended with the EDTA solution, and then the solution was added with citric acid as chelate to form a precursor solution. The molar ratio of metallic cations, EDTA and citric acid was set as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. The precursor solution was stirred for 2 h and then heated on a hot plate at 100 °C to form a wet gel, which was then heated to form a dry gel at 200–300 °C and further burned into black sponge after evaporation. The fresh powders were calcined at 1000 °C for 2 h in air to form oxidized SYT. SDC, YSZ and LSM powders were prepared by the auto-ignition process as described previously.26
1.5. The precursor solution was stirred for 2 h and then heated on a hot plate at 100 °C to form a wet gel, which was then heated to form a dry gel at 200–300 °C and further burned into black sponge after evaporation. The fresh powders were calcined at 1000 °C for 2 h in air to form oxidized SYT. SDC, YSZ and LSM powders were prepared by the auto-ignition process as described previously.26
2.2 Fabrication the RSOCs of SYT–SDC/YSZ/LSM–YSZ
The YSZ powder was dry-pressed into a green disk with a diameter of 16 mm followed by sintering in air at 1500 °C for 5 h to prepare 2 mm thick YSZ electrolyte supports. Two surfaces of the electrolyte supports were mechanically polished and ultrasonically cleaned in ethanol and distilled water. SYT and SDC powders (at a ratio 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 by mass) were mixed thoroughly with a 6 wt% ethylcellulose–terpineol binder to prepare the hydrogen electrode slurry, which was then painted on the YSZ electrolyte and sintered at 1150 °C for 3 h in air. Composite LSM–YSZ slurries were prepared by the same method, which was painted on the other side of the YSZ electrolyte and sintered at 950 °C for 3 h in air. Silver paste was printed onto both electrode surfaces for current collecting.
40 by mass) were mixed thoroughly with a 6 wt% ethylcellulose–terpineol binder to prepare the hydrogen electrode slurry, which was then painted on the YSZ electrolyte and sintered at 1150 °C for 3 h in air. Composite LSM–YSZ slurries were prepared by the same method, which was painted on the other side of the YSZ electrolyte and sintered at 950 °C for 3 h in air. Silver paste was printed onto both electrode surfaces for current collecting.
2.3 Characterization
An amount of oxidized SYT powder was treated in 100% H2 at 1400 °C for 5 h to form the reduced SYT. The X-ray diffraction (XRD, θ = 1° min−1) was performed to analyze the phase purity of the oxidized and reduced SYT, and then, XRD Rietveld refinements were performed using the GSAS software. The chemical compatibility of SYT–YSZ composite was investigated by mixing thoroughly SYT with YSZ in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio and then was sintered at 1500 °C for 5 h in air as well as SYT with SDC. Electron paramagnetic resonance (EPR) of the reduced SYT powder was recorded at 150 K to confirm the presence of high spin Ti3+. TG tests of the oxidized and reduced SYT samples were conducted using a thermal analyser at 10° min−1 in air (TGA Q5000IR). Approximately 1.5 g of SYT powder was pressed into a bar and then sintered at 1450 °C for 10 h in 100% H2, and the relative density reached 95%. The conductivity tests in reducing atmosphere were performed using a DC four-terminal method from 50 °C to 750 °C in 100% H2. The RSOCs were tested at 750 °C in a home-developed cell testing system with humidified hydrogen (3% H2O) and air as a reactant.27 The flow rate of hydrogen was 40 ml min−1. The AC impedance spectra of single cells were measured under open circuit conditions and bias voltages using an electrochemical work station (IM6e, Zahner). The measurements were conducted in the range of 1 MHz–0.1 Hz with 10 mV amplitude.
1 weight ratio and then was sintered at 1500 °C for 5 h in air as well as SYT with SDC. Electron paramagnetic resonance (EPR) of the reduced SYT powder was recorded at 150 K to confirm the presence of high spin Ti3+. TG tests of the oxidized and reduced SYT samples were conducted using a thermal analyser at 10° min−1 in air (TGA Q5000IR). Approximately 1.5 g of SYT powder was pressed into a bar and then sintered at 1450 °C for 10 h in 100% H2, and the relative density reached 95%. The conductivity tests in reducing atmosphere were performed using a DC four-terminal method from 50 °C to 750 °C in 100% H2. The RSOCs were tested at 750 °C in a home-developed cell testing system with humidified hydrogen (3% H2O) and air as a reactant.27 The flow rate of hydrogen was 40 ml min−1. The AC impedance spectra of single cells were measured under open circuit conditions and bias voltages using an electrochemical work station (IM6e, Zahner). The measurements were conducted in the range of 1 MHz–0.1 Hz with 10 mV amplitude.
3. Results and discussion
3.1 Crystal structure
Fig. 1 shows the XRD Rietveld refinement results of the oxidized and reduced SYT powder, where the strong peaks correspond to space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (221) and no additional impurities are observed. The phase structure after 100% H2 sintering at 1400 °C for 5 h was well maintained, which indicated that the SYT materials have an excellent stability under strong reducing condition. The refinement of both oxidized and reduced SYT sample gave χ2, wRp and Rp values of 5.547, 9.91% and 6.64% as well as 9.532, 14.44% and 10.6%, respectively, indicating a close fit to experimental data. After reducing, the lattice parameter (a) of SYT decreased from 3.903371 Å to 3.901100 Å, which can be due to the two combined mechanisms of the formation of oxygen vacancies and charge compensation by partial reduction of Ti4+. To further clarify the structure of the reduced SYT by the typical transmission electron microscope (TEM) image, Fig. 2(a) shows that the particle morphology of the reduced SYT particles was regular in shape and agglomerated with an average particle size in the range of 0.3–0.5 μm. The structural analysis of the HRTEM image, as shown in Fig. 2(b), further reveals that the reduced SYT particles are of high crystallinity, and the interplanar distance is clearly discerned to be 0.393 nm, corresponding to that of the (100) plane, indicating that the SYT sample calcined at high temperature and strong reducing condition is still maintaining a cubic perovskite structure.
m (221) and no additional impurities are observed. The phase structure after 100% H2 sintering at 1400 °C for 5 h was well maintained, which indicated that the SYT materials have an excellent stability under strong reducing condition. The refinement of both oxidized and reduced SYT sample gave χ2, wRp and Rp values of 5.547, 9.91% and 6.64% as well as 9.532, 14.44% and 10.6%, respectively, indicating a close fit to experimental data. After reducing, the lattice parameter (a) of SYT decreased from 3.903371 Å to 3.901100 Å, which can be due to the two combined mechanisms of the formation of oxygen vacancies and charge compensation by partial reduction of Ti4+. To further clarify the structure of the reduced SYT by the typical transmission electron microscope (TEM) image, Fig. 2(a) shows that the particle morphology of the reduced SYT particles was regular in shape and agglomerated with an average particle size in the range of 0.3–0.5 μm. The structural analysis of the HRTEM image, as shown in Fig. 2(b), further reveals that the reduced SYT particles are of high crystallinity, and the interplanar distance is clearly discerned to be 0.393 nm, corresponding to that of the (100) plane, indicating that the SYT sample calcined at high temperature and strong reducing condition is still maintaining a cubic perovskite structure.
 |
| | Fig. 1 XRD Rietveld refinement of (a) Sr0.95Y0.05TiO3−δ prepared by an EDTA–citrate complexation process calcined at 1000 °C for 2 h in air and (b) reduced in 100% H2 at 1400 °C for 5 h. | |
 |
| | Fig. 2 (a) TEM image and (b) HRTEM image of the reduced Sr0.95Y0.05TiO3−δ particles. | |
3.2 Electrical conductivity measurement
SrTiO3 based materials as potential anode materials for SOFC are highly attractive because of their ideal thermal, chemical stability and semiconducting behavior. Fig. 3 shows the electrical conductivity of the SYT sample sintered at 1450 °C for 10 h in 100% H2 and measured in 100% H2 in the temperature range of 50–750 °C. The electrical conductivity increases with increasing temperature from room-temperature to 450 °C, indicating a polaron type conduction mechanism. The SYT sample exhibited the maximum electrical conductivity of 58 S cm−1 at 450 °C. At higher temperatures, the electrical conductivity decreases, suggesting that the electrical conduction of SYT transforms from semi-conducting to free electron and ion mixed conducting behavior (a metallic-type behavior), which is still considerably larger than that of Sr0.92Y0.08TiO3 calcined and sintered both in air.19 Generally, there are two possible ways under oxidation atmosphere for the introduction of Y3+ into the Sr site of the SrTiO3 lattice. One way is that strontium vacancies will be formed by the formula given as follows:19| |
 | (1) |
 |
| | Fig. 3 The electrical conductivity of the reduced Sr0.95Y0.05TiO3−δ sample in 100% H2 in the temperature range of 50–750 °C. | |
The other is that the interstitial oxygen will be formed by the formula given as follows:25
| |
 | (2) |
Upon reducing conditions, Ti3+ ions can be formed by the electrovalent compensation.
| |
 | (3) |
This suggests that the extra electrons at the Ti sites can enter into the conduction band by the activation, leading to considerably higher electrical conductivity. Therefore, by a simple consideration of defect equilibrium in Sr0.95Y0.05TiO3−δ, the primary reaction of oxygen reduction can be represented as
| |
 | (4) |
The equilibrium constant for reaction (1) can be presented as
| |
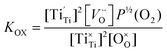 | (5) |
The site conservation and charge neutrality requirement can be expressed as
| |
 | (6) |
| |
 | (7) |
| |
 | (8) |
| |
 | (9) |
| |
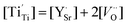 | (10) |
The solid solution formula can be described as Sr0.95Y0.05Ti0.95−2δ4+Ti2δ+0.053+O3−δ. Then, the equilibrium pressure of oxygen over Sr0.95Y0.05TiO3−δ can be expressed as
| |
 | (11) |
Under a low oxygen partial pressure, considerably more oxygen vacancies and Ti3+ from the reduction of Ti4+ can be produced. Electron-type carriers, Ti3+ (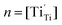 ), in SrTiO3 with doping donors were reported earlier.23,28 Therefore, the electron concentration can be expressed as:
), in SrTiO3 with doping donors were reported earlier.23,28 Therefore, the electron concentration can be expressed as:
| | |
(n − 0.05)*n2 = 4KOX (3 − δ)(0.95 − 2δ)2*P−½(O2)
| (12) |
Due to the very small value of δ, the eqn (12) can be simplified to n ∝ P−1/6(O2) dependence, which is in good agreement with previous studies.29,30 Electron concentration would increase when P(O2) decreases. Therefore, the experimental phenomenon is easy to understand where the electrical conductivity of the reduced SYT sample is considerably larger than that of SYT.
3.3 Electron paramagnetic resonance (EPR) and thermogravimetry (TG) analysis
To further confirm the presence of Ti3+ in the reduced SYT sample, electron paramagnetic resonance (EPR) was performed at 150 K to determine the presence of unpaired electrons of Ti3+ in the reduced SYT sample. As shown in Fig. 4(a), the reduced SYT sample showed a strong EPR signal at g = 1.958, characteristic of paramagnetic Ti3+ ions, directly proving the existence of Ti3+ species accompanied by the formation of oxygen vacancies.31–33 In order to estimate the oxygen vacancy concentration of the reduced SYT sample, the weight changes measured by TG tests with the oxidized and reduced SYT samples were carried out in air, as shown in Fig. 4(b). Supposing that the contents of variable oxygen vacancies of the oxidized SYT sample were almost filled by the environmental oxygen, then the oxygen vacancy concentration of the reduced SYT sample can be approximately estimated. With the increasing temperatures, the oxidized SYT sample exhibited a series of weight losses, which mainly resulted from the lattice oxygen desorption with an oxygen desorption rate, and the assumption of reaction (13) is a dynamic equilibrium with an oxygen absorption rate as follows:34,35| |
 | (13) |
 |
| | Fig. 4 (a) Electron paramagnetic resonance (EPR) of reduced Sr0.95Y0.05TiO3−δ powder conducted at the temperature of 150 K. (b) TG tests of oxidized and reduced Sr0.95Y0.05TiO3−δ in air at 1200 °C at the rate of 10 °C min−1. | |
The higher the temperature, the shorter the relaxation time. As the temperature increases, the reduced SYT sample reached a maximum weight at 1091 °C, which was then followed by a weight loss, showing the same trend as reported in previous studies.36 Because the reduced SYT sample was restored to form a large number of oxygen vacancies and Ti3+ species, with the increasing temperatures in air, the reduced SYT sample must absorb the environmental oxygen with an oxygen absorption rate, which must be higher than the oxygen desorption rate below 1091 °C. While the oxygen absorption rate was equal to the oxygen desorption rate around 1091 °C, the maximum weight for the reduced SYT sample was observed. Compared to the weight changes between oxidized and reduced SYT around this temperature, the variable oxygen vacancy concentration of the reduced SYT sample was estimated to be 0.03365 because of the negligible weight change for the Ti3+ species oxidation, and then the total concentration of Ti3+ species was calculated to be 0.1173. In this case, the reduced SYT can be described as Sr0.95Y0.05Ti0.88274+Ti0.11733+O2.96635.
3.4 Electrochemical performances of RSOCs
Fig. 5 shows the voltage–current curves of RSOCs measured at 750 °C using 2 mm thick YSZ as the electrolyte and SYT–SDC as the hydrogen electrode with humidified hydrogen (3% H2O) as a reactant. The open circuit voltage (OCV) corresponding to zero current density was 0.9 V, which could be influenced by the steam/hydrogen ratio and temperature.37 The positive current density and the negative current density were observed with applied voltages to the SOFC mode and the SOEC mode, respectively. From the voltage–current curves, there is a smooth transition across OCV from the SOFC mode to the SOEC mode, indicating that the SYT–SDC/YSZ/LSM–YSZ cells are reversible for the charge transfer reaction. The discharging and charging current densities were 18 mA cm−2 at 0.6 V and −23 mA cm−2 at 1.2 V, which showed a considerably better electrochemical performance under the SOEC mode.
 |
| | Fig. 5 Voltage–current density curves of RSOCs measured at 750 °C using 2 mm thick YSZ as the electrolyte and SYT–SDC as the hydrogen electrode with humidified hydrogen (3% H2O) as a reactant. | |
To further investigate the electrochemical process of RSOCs with SYT–SDC as hydrogen electrode at a variation of applied voltages, AC impedance spectroscopy was performed under the SOFC mode and the SOEC mode, as shown in Fig. 6(a) and (b). In these spectra, the intercepts with the real axis at low frequencies represent the total cell resistance (Rt) of the cell and the value of the intercept at high frequency is bulk resistance (Ro), including the ohmic resistance of the YSZ electrolyte, the ohmic resistance of porous electrodes and contact resistances of both electrodes, whereas the difference of the two values corresponds to the electrode polarization resistance (Rp), including the polarization losses of the hydrogen electrode and air electrode. The Ro and Rp values with different applied voltages were measured at 750 °C, as shown in Fig. 7(a), for example at OCV, the Ro and Rp values are 9.06 Ω cm2 and 10.57 Ω cm2, respectively. The relationship between the resistance and the applied voltages (absolute values) can also be reflected from the results. It is observed that the Ro values had small amount of changes with small slopes at both the SOFC (−0.29 Ω cm2 V−1) and SOEC mode (0.5 Ω cm2 V−1) and a sudden decrease from the SOFC mode to SOEC mode, whereas the Rp values decreased all the time with the increasing potentials both at the SOFC (−2.59 Ω cm2 V−1) and SOEC mode (−9.65 Ω cm2 V−1) and also had a sudden turning point at the OCV, which indicated that the electrode performance of the SYT–SDC hydrogen electrode can be improved under higher applied voltages. For a better understanding, the Ro and Rp values change under the SOFC mode and the SOEC mode, and the reaction zone of the mixed-conducting hydrogen electrodes can extend from the three phase boundary (TPB) to the gas/electrode interface through the chemical diffusion in the hydrogen electrode. The difference of the electrochemical potential is the driving force for the electrode reaction, and Fig. 8(a) shows the tendency of the local P(O2) changes of the hydrogen electrode under the SOFC mode and the SOEC mode. The local P(O2) can slightly increase under the SOFC mode and reduce under the SOEC mode.38 According to the conductor mechanism of SrTiO3-based materials,29,30 the SYT-based hydrogen electrode can show better conductivity under SOEC mode than that under SOFC mode, as shown in Fig. 8(b), indicating that the electrical conductivity and electro-catalytic property can be improved.
 |
| | Fig. 6 In situ AC impedance of RSOCs at 750 °C with external applied voltages (a) under SOFC mode and (b) under SOEC mode. | |
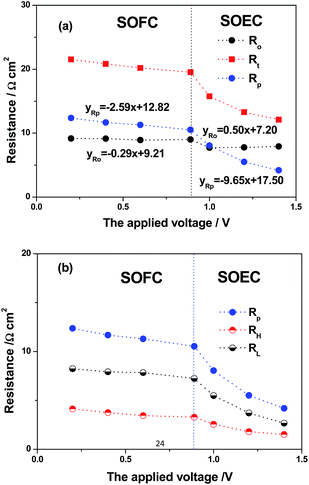 |
| | Fig. 7 (a) The total cell resistance (Rt), bulk resistance (Ro), the electrode polarization resistance (Rp) and (b) high and low frequency polarization resistances (RH and RL) at 750 °C with the different applied voltages. | |
 |
| | Fig. 8 (a) Schematic of the local P(O2) change of the SYT–SDC hydrogen electrode and (b) the variation of electrical conductivity in SrTiO3-based materials depending on the local P(O2) under SOFC mode and under SOEC mode. | |
To investigate the decreasing Rp values, two depressed arcs were observed for each impedance spectrum from Fig. 6, indicating two rate-limiting steps, where RH and RL represent high and low frequency polarization resistances, respectively, as shown in Fig. 7(b). The high-frequency arc was attributed to the polarization during the charge-transfer process of the electrode, whereas the low-frequency was due to the adsorption and desorption on the electrode surface and the diffusion of oxygen and hydrogen species.39,40 The decrease of RH values from the SOFC to SOEC mode means considerably better charge-transfer process of the electrode due to the increasing conductivity of the SYT–SDC hydrogen electrode, whereas the decrease of RL from the SOFC to SOEC mode resulted from the improved LSM–YSZ oxygen electrode with an oxidizing atmosphere and electro-catalytic property of SYT–SDC hydrogen electrode for the steam electrolysis.
3.5 Stability test of RSOCs with SYT–SDC hydrogen electrode
Fig. 9(a) shows the chemical compatibility of SYT–YSZ composites, investigated by thoroughly mixing SYT with YSZ in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio, and then sintering at 1500 °C for 5 h in air as well as SYT with SDC. There are no new peaks identifiable or shift of XRD peaks in the patterns, indicating that there are no significant reactions between SYT and others. Although interfacial reaction phenomenon between the La-doped SrTiO3 and 1CeO2–10Sc2O3–89ZrO2 (ScSZ) electrolyte with the La2Zr2O7 impurity phase was observed,41 the short-term performance of RSOCs with the SYT–SDC hydrogen electrode at 750 °C shows a stable electrochemical process as shown in Fig. 9(b), where the current density reached −23.6 mA cm−2 with the applied electrical voltage of 1.2 V, indicating that SYT–SDC can be a potential hydrogen electrode for RSOCs.
1 weight ratio, and then sintering at 1500 °C for 5 h in air as well as SYT with SDC. There are no new peaks identifiable or shift of XRD peaks in the patterns, indicating that there are no significant reactions between SYT and others. Although interfacial reaction phenomenon between the La-doped SrTiO3 and 1CeO2–10Sc2O3–89ZrO2 (ScSZ) electrolyte with the La2Zr2O7 impurity phase was observed,41 the short-term performance of RSOCs with the SYT–SDC hydrogen electrode at 750 °C shows a stable electrochemical process as shown in Fig. 9(b), where the current density reached −23.6 mA cm−2 with the applied electrical voltage of 1.2 V, indicating that SYT–SDC can be a potential hydrogen electrode for RSOCs.
 |
| | Fig. 9 (a) Chemical compatibility of SYT–YSZ and SYT–SDC composites after sintering at 1500 °C for 5 h. (b) The short-term performance of RSOCs with the SYT–SDC hydrogen electrode with an applied electrical voltage of 1.2 V at 750 °C. | |
4. Conclusion
In this work, Sr0.95Y0.05TiO3−δ (SYT) was synthesized and characterized as the hydrogen electrode of oxygen-ion conducting RSOCs. XRD Rietveld refinement, electron paramagnetic resonance and TG together demonstrated that the solid solution formula of the reduced SYT can be described as Sr0.95Y0.05Ti0.88274+Ti0.11733+O2.96635. The reduced SYT was a typical n-type conductor with a total conductivity of 58 S cm−1 at 450 °C. RSOCs with the cell configuration of SYT–SDC/YSZ/LSM–YSZ were assembled and evaluated. The electrical conductivity and the electro-catalytic property of the SYT-based hydrogen electrode could be improved under the SOFC mode. A stable steam electrolysis process was indicated by the short-term cell performance, where the current density reached 23.6 mA cm−2 at the applied voltage of 1.2 V at 750 °C, indicating that SYT–SDC is a potential hydrogen electrode.
Acknowledgements
The authors wish to thank Japan Society for the Promotion of Science (JSPS) for financial support through a Post-doctoral Fellowship for Foreign Researchers and the financial support from Chinese Natural Science Foundation on contract no. 51102107.
Notes and references
- M. A. Laguna-Bercero, J. Power Sources, 2012, 203, 4 CrossRef CAS PubMed.
- M. Ni, Int. J. Hydrogen Energy, 2009, 34, 7795 CrossRef CAS PubMed.
- A. Hauch, S. D. Ebbesen, S. H. Jensen and M. Mogensen, J. Mater. Chem., 2008, 18, 2331 RSC.
- F. He, D. Song, R. R. Peng, G. Y. Meng and S. F. Yang, J. Power Sources, 2010, 195, 3359 CrossRef CAS PubMed.
- K. F. Chen and S. P. Jiang, Int. J. Hydrogen Energy, 2011, 36, 10541 CrossRef CAS PubMed.
- M. Keane, M. K. Mahapatra, A. Verma and P. Singh, Int. J. Hydrogen Energy, 2012, 37, 16776 CrossRef CAS PubMed.
- A. Hauch, S. H. Jensen, J. B. Bilde-Sørensen and M. Mogensen, J. Electrochem. Soc., 2007, 154, A619 CrossRef CAS PubMed.
- A. Hauch, S. D. Ebbesen, S. H. Jensen and M. Mogensen, J. Electrochem. Soc., 2008, 155, B1184 CrossRef CAS PubMed.
- L. Holzer, B. Iwanschitz, T. Hocker, B. Münch, M. Prestat, D. Wiedenmann, U. Vogt, P. Holtappels, J. Sfeir, A. Mai and T. Graule, J. Power Sources, 2011, 196, 1279 CrossRef CAS PubMed.
- J. Sehested, J. A. P. Gelten, I. N. Remediakis, H. Bengaard and J. K. Nørskov, J. Catal., 2004, 223, 432 CrossRef CAS PubMed.
- X. Yang and J. T. S. Irvine, J. Mater. Chem., 2008, 18, 2349 RSC.
- S. W. Tao, J. T. S. Irvine and J. A. Kilner, Adv. Mater., 2005, 17, 1734 CrossRef CAS.
- S. P. Jiang, L. Zhang and Y. J. Zhang, J. Mater. Chem., 2007, 17, 2627 RSC.
- G. Kim, G. Corre, J. T. S. Irvine, J. M. Vohs and R. J. Gorte, Electrochem. Solid-State Lett., 2008, 11, B16 CrossRef CAS PubMed.
- S. S. Xu, S. S. Li, W. T. Yao, D. H. Dong and K. Xie, J. Power Sources, 2013, 230, 115 CrossRef CAS PubMed.
- D. Neagu and J. T. S. Irvine, Chem. Mater., 2010, 22, 5042 CrossRef CAS.
- J. Canales-Vazquez, M. J. Smith, J. T. S. Irvine and W. Z. Zhou, Adv. Funct. Mater., 2005, 15, 1000 CrossRef CAS.
- S. Li, Y. X. Li, Y. Gan, K. Xie and G. Y. Meng, J. Power Sources, 2012, 218, 244 CrossRef CAS PubMed.
- X. Huang and H. Zhao, J. Phys. Chem. Solids, 2006, 67, 2609 CrossRef CAS PubMed.
- S. Koutcheiko, Y. Yoo, A. Petric and I. Davidson, Ceram. Int., 2006, 32, 67 CrossRef CAS PubMed.
- Y. Li, J. Zhou, D. Dong, Y. Wang, J. Z. Jiang, H. Xiang and K. Xie, Phys. Chem. Chem. Phys., 2012, 14, 15547 RSC.
- O. A. Marina, N. L. Canfield and J. W. Stevenson, Solid State Ionics, 2002, 149, 21 CrossRef CAS.
- D. Burnat, A. Heel, L. Holzer, D. Kata, J. Lis and T. Graule, J. Power Sources, 2012, 201, 26 CrossRef CAS PubMed.
- H. S. Kim, S. P. Yoon, J. W. Yun, S. A. Song, S. C. Jang, S. W. Nam and Y. G. Shul, Int. J. Hydrogen
Energy, 2012, 37, 16130 CrossRef CAS PubMed.
- Q. L. Ma, F. Tietz and D. Stover, Solid State Ionics, 2011, 192, 535 CrossRef CAS PubMed.
- Y. H. Ling, J. Chen, Z. B. Wang, C. R. Xia, R. R. Peng and Y. L. Lu, Int. J. Hydrogen Energy, 2013, 38, 7430 CrossRef CAS PubMed.
- L. Zhao, B. B. He, Y. H. Ling, Z. Q. Xun, R. R. Peng, G. Y. Meng and X. Q. Liu, Int. J. Hydrogen Energy, 2010, 35, 3769 CrossRef CAS PubMed.
- X. Li, H. L. Zhao, W. Shen, F. Gao, X. L. Huang, Y. Li and Z. M. Zhu, J. Power Sources, 2007, 166, 47 CrossRef CAS PubMed.
- D. J. Cumming, V. V. Kharton, A. A. Yaremchenko, A. V. Kovalevsky and J. A. Kilner, J. Am. Ceram. Soc., 2011, 94(9), 2993 CrossRef CAS PubMed.
- E. Niwa, K. Sato, K. Yashiro and J. Mizusaki, ECS Trans., 2009, 25(2), 2631 Search PubMed.
- S. V. Chong, J. Xia, N. Suresh, K. Yamaki and K. Kadowaki, Solid State Commun., 2008, 148, 345 CrossRef CAS PubMed.
- J. Soria, J. Sanz, I. Sobrados, J. M. Coronado, F. Fresno and M. D. Hernández-Alonso, Catal. Today, 2007, 129, 240 CrossRef CAS PubMed.
- M. D. Glinchuk, I. P. Bykov, A. M. Slipenyuk, V. V. Laguta and L. Jastrabik, Phys. Solid State, 2001, 43, 841 CrossRef CAS.
- L. W. Tai, M. M. Nasrallah and H. U. Anderson, Solid State Ionics, 1995, 76, 259 CrossRef CAS.
- Y. H. Ling, L. Zhao, B. Lin, Y. C. Dong, X. Z. Zhang, G. Y. Meng and X. Q. Liu, Int. J. Hydrogen Energy, 2010, 35, 6905 CrossRef CAS PubMed.
- X. Li, H. L. Zhao, F. Gao, Z. M. Zhu, N. Chen and W. Shen, Solid State Ionics, 2008, 179, 1588 CrossRef CAS PubMed.
- C. Jin, C. H. Yang, F. Zhao, D. Cui and F. L. Chen, Int. J. Hydrogen Energy, 2011, 36, 6905 Search PubMed.
- T. Kawada, T. Watanabe, A. Kaimai, K. Kawamura, Y. Nigara and J. Mizusaki, Solid State Ionics, 1998, 108, 391 CrossRef CAS.
- S. Li, Z. Lu, X. Huang, B. Wei and W. Su, J. Phys. Chem. Solids, 2007, 68, 1707 CrossRef CAS PubMed.
- Y. H. Ling, X. Z. Zhang, S. W. Wang, L. Zhao, B. Lin and X. Q. Liu, J. Power Sources, 2010, 295, 7042 CrossRef PubMed.
- G. Chen, H. Kishimoto, K. Yamaji, K. Kuramoto and T. Horita, J. Power Sources, 2014, 246, 49 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. The precursor solution was stirred for 2 h and then heated on a hot plate at 100 °C to form a wet gel, which was then heated to form a dry gel at 200–300 °C and further burned into black sponge after evaporation. The fresh powders were calcined at 1000 °C for 2 h in air to form oxidized SYT. SDC, YSZ and LSM powders were prepared by the auto-ignition process as described previously.26
1.5. The precursor solution was stirred for 2 h and then heated on a hot plate at 100 °C to form a wet gel, which was then heated to form a dry gel at 200–300 °C and further burned into black sponge after evaporation. The fresh powders were calcined at 1000 °C for 2 h in air to form oxidized SYT. SDC, YSZ and LSM powders were prepared by the auto-ignition process as described previously.26
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 by mass) were mixed thoroughly with a 6 wt% ethylcellulose–terpineol binder to prepare the hydrogen electrode slurry, which was then painted on the YSZ electrolyte and sintered at 1150 °C for 3 h in air. Composite LSM–YSZ slurries were prepared by the same method, which was painted on the other side of the YSZ electrolyte and sintered at 950 °C for 3 h in air. Silver paste was printed onto both electrode surfaces for current collecting.
40 by mass) were mixed thoroughly with a 6 wt% ethylcellulose–terpineol binder to prepare the hydrogen electrode slurry, which was then painted on the YSZ electrolyte and sintered at 1150 °C for 3 h in air. Composite LSM–YSZ slurries were prepared by the same method, which was painted on the other side of the YSZ electrolyte and sintered at 950 °C for 3 h in air. Silver paste was printed onto both electrode surfaces for current collecting.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio and then was sintered at 1500 °C for 5 h in air as well as SYT with SDC. Electron paramagnetic resonance (EPR) of the reduced SYT powder was recorded at 150 K to confirm the presence of high spin Ti3+. TG tests of the oxidized and reduced SYT samples were conducted using a thermal analyser at 10° min−1 in air (TGA Q5000IR). Approximately 1.5 g of SYT powder was pressed into a bar and then sintered at 1450 °C for 10 h in 100% H2, and the relative density reached 95%. The conductivity tests in reducing atmosphere were performed using a DC four-terminal method from 50 °C to 750 °C in 100% H2. The RSOCs were tested at 750 °C in a home-developed cell testing system with humidified hydrogen (3% H2O) and air as a reactant.27 The flow rate of hydrogen was 40 ml min−1. The AC impedance spectra of single cells were measured under open circuit conditions and bias voltages using an electrochemical work station (IM6e, Zahner). The measurements were conducted in the range of 1 MHz–0.1 Hz with 10 mV amplitude.
1 weight ratio and then was sintered at 1500 °C for 5 h in air as well as SYT with SDC. Electron paramagnetic resonance (EPR) of the reduced SYT powder was recorded at 150 K to confirm the presence of high spin Ti3+. TG tests of the oxidized and reduced SYT samples were conducted using a thermal analyser at 10° min−1 in air (TGA Q5000IR). Approximately 1.5 g of SYT powder was pressed into a bar and then sintered at 1450 °C for 10 h in 100% H2, and the relative density reached 95%. The conductivity tests in reducing atmosphere were performed using a DC four-terminal method from 50 °C to 750 °C in 100% H2. The RSOCs were tested at 750 °C in a home-developed cell testing system with humidified hydrogen (3% H2O) and air as a reactant.27 The flow rate of hydrogen was 40 ml min−1. The AC impedance spectra of single cells were measured under open circuit conditions and bias voltages using an electrochemical work station (IM6e, Zahner). The measurements were conducted in the range of 1 MHz–0.1 Hz with 10 mV amplitude.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (221) and no additional impurities are observed. The phase structure after 100% H2 sintering at 1400 °C for 5 h was well maintained, which indicated that the SYT materials have an excellent stability under strong reducing condition. The refinement of both oxidized and reduced SYT sample gave χ2, wRp and Rp values of 5.547, 9.91% and 6.64% as well as 9.532, 14.44% and 10.6%, respectively, indicating a close fit to experimental data. After reducing, the lattice parameter (a) of SYT decreased from 3.903371 Å to 3.901100 Å, which can be due to the two combined mechanisms of the formation of oxygen vacancies and charge compensation by partial reduction of Ti4+. To further clarify the structure of the reduced SYT by the typical transmission electron microscope (TEM) image, Fig. 2(a) shows that the particle morphology of the reduced SYT particles was regular in shape and agglomerated with an average particle size in the range of 0.3–0.5 μm. The structural analysis of the HRTEM image, as shown in Fig. 2(b), further reveals that the reduced SYT particles are of high crystallinity, and the interplanar distance is clearly discerned to be 0.393 nm, corresponding to that of the (100) plane, indicating that the SYT sample calcined at high temperature and strong reducing condition is still maintaining a cubic perovskite structure.
m (221) and no additional impurities are observed. The phase structure after 100% H2 sintering at 1400 °C for 5 h was well maintained, which indicated that the SYT materials have an excellent stability under strong reducing condition. The refinement of both oxidized and reduced SYT sample gave χ2, wRp and Rp values of 5.547, 9.91% and 6.64% as well as 9.532, 14.44% and 10.6%, respectively, indicating a close fit to experimental data. After reducing, the lattice parameter (a) of SYT decreased from 3.903371 Å to 3.901100 Å, which can be due to the two combined mechanisms of the formation of oxygen vacancies and charge compensation by partial reduction of Ti4+. To further clarify the structure of the reduced SYT by the typical transmission electron microscope (TEM) image, Fig. 2(a) shows that the particle morphology of the reduced SYT particles was regular in shape and agglomerated with an average particle size in the range of 0.3–0.5 μm. The structural analysis of the HRTEM image, as shown in Fig. 2(b), further reveals that the reduced SYT particles are of high crystallinity, and the interplanar distance is clearly discerned to be 0.393 nm, corresponding to that of the (100) plane, indicating that the SYT sample calcined at high temperature and strong reducing condition is still maintaining a cubic perovskite structure.






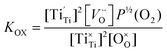




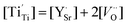

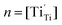 ), in SrTiO3 with doping donors were reported earlier.23,28 Therefore, the electron concentration can be expressed as:
), in SrTiO3 with doping donors were reported earlier.23,28 Therefore, the electron concentration can be expressed as:


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio, and then sintering at 1500 °C for 5 h in air as well as SYT with SDC. There are no new peaks identifiable or shift of XRD peaks in the patterns, indicating that there are no significant reactions between SYT and others. Although interfacial reaction phenomenon between the La-doped SrTiO3 and 1CeO2–10Sc2O3–89ZrO2 (ScSZ) electrolyte with the La2Zr2O7 impurity phase was observed,41 the short-term performance of RSOCs with the SYT–SDC hydrogen electrode at 750 °C shows a stable electrochemical process as shown in Fig. 9(b), where the current density reached −23.6 mA cm−2 with the applied electrical voltage of 1.2 V, indicating that SYT–SDC can be a potential hydrogen electrode for RSOCs.
1 weight ratio, and then sintering at 1500 °C for 5 h in air as well as SYT with SDC. There are no new peaks identifiable or shift of XRD peaks in the patterns, indicating that there are no significant reactions between SYT and others. Although interfacial reaction phenomenon between the La-doped SrTiO3 and 1CeO2–10Sc2O3–89ZrO2 (ScSZ) electrolyte with the La2Zr2O7 impurity phase was observed,41 the short-term performance of RSOCs with the SYT–SDC hydrogen electrode at 750 °C shows a stable electrochemical process as shown in Fig. 9(b), where the current density reached −23.6 mA cm−2 with the applied electrical voltage of 1.2 V, indicating that SYT–SDC can be a potential hydrogen electrode for RSOCs.





