Experimental study and thermodynamic modeling for purification of extracted algal lipids using an organic/aqueous two-phase system
Abstract
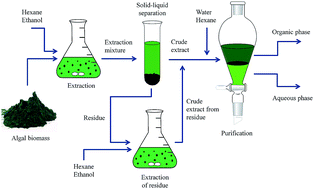
The extraction and purification of lipids from the microalgae Chlorella vulgaris have been investigated. First, a mixture of hexane and ethanol was used to extract lipids from the algal biomass. Ultrasonication was employed to disrupt the cell wall and increase the extraction performance. Under these conditions, over 90% of the fatty acids in the biomass were extracted. Second, a biphasic system was formed by adding water and hexane to the extracted crude oil. In this way, fatty acids were mainly distributed in the organic phase (mostly hexane and ethanol) while most of the contaminants remained in the aqueous phase (mostly water and ethanol). Equilibrium distribution data between the phases were obtained to investigate the fatty acid lost to the aqueous phase during the purification process. The results showed that adding more water and hexane to the extraction mixture leads to a greater phase separation, as well as to higher purification of the extracted lipids. However purification efficiency did not improve very much if hexane and water were added at more than their optimum value. Two thermodynamic models (UNIQUAC and NRTL) were used in order to describe the partitioning behaviour of fatty acids in this system. The results indicated that these models can accurately estimate the fatty acid partition coefficient with average absolute deviation percentage (AAD%) of 8.69 and 9.46 for the UNIQUAC and the NRTL models, respectively. The AAD% of fatty acid recovery yield was 4.91 and 5.60 for the UNIQUAC and the NRTL models, respectively.

 Please wait while we load your content...
Please wait while we load your content...