Tuning the work function of polyaniline via camphorsulfonic acid: an X-ray photoelectron spectroscopy investigation
Abstract
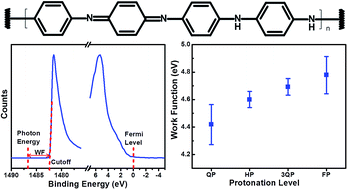
In this work, we present the first demonstration of tuning the work function of polyaniline by controlling the concentration level of camphorsulfonic acid as a protonic acid dopant and m-cresol as a solvent. Optical, thermal, structural, and electronic properties, along with surface topography and elemental analysis of protonated polyaniline, were studied in detail to investigate the effect of camphorsulfonic acid on the work function of polyaniline. The results showed that an increase in camphorsulfonic acid content induces a gradual transformation in the polyaniline structure from an emeraldine base to an emeraldine salt phase, which is associated with an increase in electrical conductivity and an improvement in crystallinity. X-ray photoelectron spectroscopy was used to evaluate the work function and to determine the elemental composition of the surface and several atomic layers beneath the surface. The results showed that increasing the camphorsulfonic acid content from quarter protonated to fully protonated leads to an increase in the work function of polyaniline from 4.42 ± 0.14 eV to 4.78 ± 0.13 eV.

 Please wait while we load your content...
Please wait while we load your content...