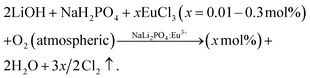A new high sensitivity Na2LiPO4:Eu OSL phosphor
P. D. Sahare*a,
Manveer Singha and
Pratik Kumarb
aDepartment of Physics & Astrophysics, University of Delhi, Delhi–110 007, India. E-mail: pdsahare@physics.du.ac.in; pdsahare@yahoo.co.in; Fax: +91-11-2766-7061; Tel: +91-11-2766-7793
bMedical Physics Unit, BRAIRCH, AIIMS, Ansari Nagar, New Delhi–110 029, India
First published on 27th November 2014
Abstract
A new high sensitivity Na2LiPO4:Eu optically stimulated luminescence dosimeter (OSLD) material was prepared by a simple solid-state diffusion method. The formation of the material was confirmed by comparing the experimental data with that available in the literature (JCPDF # 80-2110). The dosimetric properties of the phosphor material using a continuous wave-optically stimulated luminescence (CW-OSL) technique were studied. The material was studied for different concentrations of the impurity and also for different heating treatments. The material doped with 1.0 mol% and annealed at 873 K was found to be the most sensitive. The phosphor was found to have all the good dosimetric characteristics, such as tissue equivalence (low-Z, Zeff ∼ 10.8), high sensitivity (∼3 times less than the commercially available Al2O3:C, Landauer Inc., USA and BeO, Thermalox® 995, Materion Inc., USA), low fading (∼6.2% in 40 days), wide range of dose response (0.1–1.0 kGy), excellent reusability, easy optical bleaching (annealing) for its reuse, etc., which makes the material useful for dosimetry of high-energy radiations using OSL. The advantages of our OSLD phosphor are the easily available and inexpensive ingredients and a very simple method of preparation that makes it cost effective as compared to the commercially available OSLD phosphors. Also, it is easy to handle, unlike the Thermalox® 995 (BeO) dosimeters which are very toxic, and require a special method of preparation and handling.
Introduction
Optically stimulated luminescence (OSL) has established itself as an improved and more reliable technique for dosimetry of high-energy radiations. The technique is becoming more and more popular because of several advantages over thermoluminescence (TL) dosimetry, such as the completely optical nature of the instrumentation, possibility of online (in situ) measurements using optical fiber, low power consuming LED sources for stimulation, facility of reestimation of radiation doses in case of any doubts and, over and above all, no structural changes1 due to heating that lose its reusability, unlike in some TLD phosphor materials.2–5 However, there is not much choice due to the paucity of commercially available OSL phosphors and the high fading in others. The OSL phosphors available are also costly due to their difficult methods of synthesis. For example, for producing Al2O3:C,6,7 one needs to have a very high temperature vacuum furnace as the doping of carbon is not possible in an atmospheric environment; for producing another OSL phosphor, BeO,8 special arrangements are needed for its production and handling as it is very toxic to human beings.The general requirement of a good OSL phosphor is that the emission should be between 350 and 425 nm (where most common detectors are most sensitive) and the defects (traps) should have a high photo-ionization cross-section in the blue-green region (450–550 nm) or IR region (650–800 nm). The sensitivity of an OSLD phosphor depends on the kind of defects, stimulation sources and availability of suitable filters and on the detector (generally a wide-band PMT). The tissue equivalent (low-Z) phosphors are much preferred due to their flat energy response and their suitability to be used in a mixed radiation field. Attempts are, therefore, going on to study new (preferably low-Z) materials for their applications as OSL phosphors, such as, LiMgPO4:Tb,B,9 Na2SiF6:Cu,P,10 Al2O3:B,11 Y3Al5O12:C,12 Cu-doped quartz (SiO2:Cu),13 LiAlO2,14 MgO:Tb,15 NaMgF3:Eu,16 etc. However, all these phosphors are at the developmental level and the issues related to their sensitivity and fading need to be addressed before they are finally accepted as OSLD phosphors. More details and a comparative study can be seen in some recent review papers.17–19
In the present paper we report optically stimulated luminescence (OSL) and thermoluminescence (TL) studies of a new high sensitivity low-Z Na2LiPO4:Eu phosphor for its application for OSL dosimetry. Na2LiPO4:Eu is really a multifunctional advanced ceramic as it has already been proved to be a red phosphor for solid-state lighting and display applications20 and a high-sensitivity TLD phosphor for dosimetry applications.21 It was intuitive to study this material for its application as an OSL phosphor and it proved to be satisfying. The phosphor Na2LiPO4:Eu is found to have several ‘good’ characteristics, such as low-Z material, very easy method of preparation, nontoxic in nature, highly sensitive, linear OSL dose response over a wide range, low fading, excellent reusability, emission well separated (peaking at around 420 nm) from that of the excitation source (470 nm blue LEDs). Phenomenological studies have also been done to understand the process of OSL and a model for trapping and emptying and recombining the traps with luminescence (hole) centers has been proposed.
Result and discussions
XRD analysis
XRD patterns of the NaLi2PO4:Eu3+ phosphor are as shown in Fig. 1. The peak positions in the diffraction pattern of the synthesized material were indexed by comparing them with the standard data available in the literature (JCPDF # 80-2110)21 and found to be in agreement, confirming the formation of the material. All the XRD peaks are indexed (hkl) for different lattice planes. The material is found to be in the orthorhombic crystal system, with the space group Pmnb (62).22 The stick pattern of the standard data (JCPDF # 80-2110) along with the experimental one has also been given for a ready reference. No separate peaks corresponding to any impurity phase were observed at low concentrations (0.05–0.1 mol%), showing that the impurity has a good solubility in the matrix in this range. However, when the impurity concentration was increased beyond this range (<0.2 mol%) new peaks corresponding to impurity clusters of monoclinic phase of Eu2O3 (JCPDF # 43-1009) were observed. This may be attributed to precipitation of the impurity ions and forming clusters. Consequently, it has also been observed that the cell parameters and the cell volumes of the samples gradually decreased with the impurity concentration due to a decrease in the crystallinity of the materials with the addition of the Eu2O3 phase.23 This is very important from the application point of view as the precipitation of impurity clusters and/or the stress/strain developed due to changes in the cell volume could change the luminescence characteristics of the phosphor material.24 The details of the study can be found in our earlier paper.25 | ||
| Fig. 1 XRD pattern of NaLi2PO4:Eu based on our experimental data. The stick pattern of the XRD pattern plotted using the data available in the literature (JCPDF file # 80-2110)21 is also given for comparison. | ||
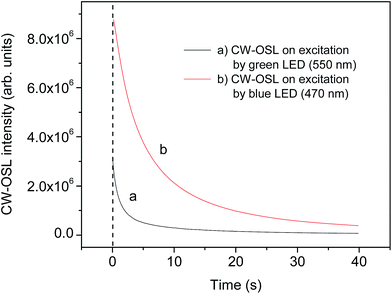
OSL measurements
| OSL component | OSL response stimulated by green LED (570 nm) | OSL response stimulated by blue LED (470 nm) | ||||
|---|---|---|---|---|---|---|
| Coefficients | Decay constant (s) | Photo-ionization cross-section σ (cm2) | Coefficients | Decay constant (s) | Photo-ionization cross-section σ (cm2) | |
| Fast | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 656.8 (A1) 656.8 (A1) |
1.13 (τ1) | 0.82 × 10−17 | 191![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 734.5 (A1) 734.5 (A1) |
3.45 (τ1) | 0.15 × 10−17 |
| Medium | 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 279.3 (A2) 279.3 (A2) |
0.10 (τ2) | 0.91 × 10−16 | — | — | — |
| Slow | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103.1 (A3) 103.1 (A3) |
11.69 (τ3) | 0.8 × 10−18 | 440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 293.7 (A2) 293.7 (A2) |
0.50 (τ2) | 0.11 × 10−16 |
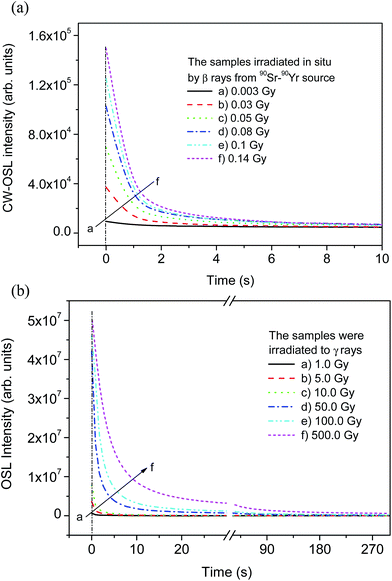
Dose response
Na2LiPO4:Eu samples annealed at 873 K for 1 h were irradiated at low doses (in the range of 0.003–0.14 Gy) of β rays from the 90Sr–90Yr source and the CW-OSL decay curves were recorded. The results are as shown in Fig. 5(a) and (b). For high doses, however, the samples were irradiated with different doses of γ rays from a 137Cs source. No difference in the shape of the decay curve was observed except for the increase in intensity with the dose. The response curves for stimulation by both the blue and green LED lights, i.e., 470 and 530 nm, respectively, are as shown in Fig. 6. From the figure it can be seen that both response curves are very linear in the dose range (i.e., 0.05–10 Gy). However, beyond this range (10 Gy–100 Gy) they show supralinearity before saturation. No further studies, therefore, were done thereafter. These results show that there is little effect between the different types of radiations (i.e. electrons and photons) and their energies due to the low effective atomic number (Zeff ≈ 10.8). The dose response is found to be within the same range as in the case of the commercially available Al2O3:C (Landauer, USA) OSL phosphor. Several batches of the material for the optimized impurity concentration to see the batch to batch variation and were given proper annealing treatments. No appreciable variation was observed in their decay curves and dose response.Correlation of CW-OSL with TL peaks and supralinearity of the dose response
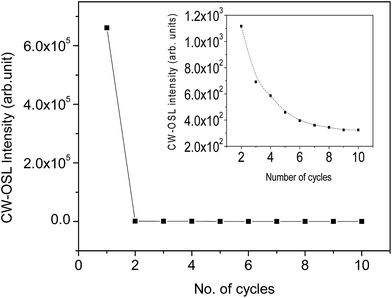
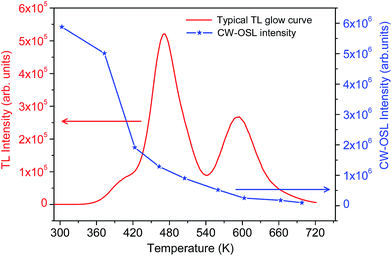
The CW-OSL dose response of Na2LiPO4:Eu phosphor is shown in Fig. 6. It can be seen in the figure that the dose response is supralinear in the dose range 10.0–100.0 Gy. To understand the phenomenon in more detail, some more experiments were performed. It was found that in CW-OSL the intensity is reduced by almost three orders of magnitude after the first readout (Fig. 7). However, it can be seen (inset of Fig. 7) that the CW-OSL intensity does not completely diminish even after repeated readouts or by optical annealing. This indicates some optically sensitive traps (though negligibly small in numbers) still remain inside the material and get depleted on subsequent readouts. The material was optically annealed for half an hour but still some (though very weak) OSL signal was observed. In order to investigate which of the TL peaks are contributing to the CW-OSL in our material, the irradiated sample was subjected to thermal bleaching at different temperatures. For this, the NaLi2PO4:Eu sample was irradiated with beta radiation of 0.1 Gy each time and heated with a heating rate of 5 K s−1 on metal strip (in the Riso TL/OSL Reader) up to 373, 423, 463, 505, 560, 603, 663 and 698 K, held for 30 s, cooled to room temperature rapidly by switching off the heater and then the CW-OSL was recorded.30 These results were plotted and are shown in Fig. 8. The results show that all the peaks are OSL sensitive. To confirm these results further, the phosphor material was irradiated by β rays, different TL peaks were cleaned by a thermal cleaning method and the intensity of the CW-OSL decay curves was plotted with the temperature up to which the TL glow curves were recorded before taking the CW-OSL. The results are as shown in Fig. 9. It can be seen that OSL intensity was observed even after cleaning the first two peaks and even the third peak partially. It seems that some of the deeper traps were transferred to the vacancies created by thermal cleaning on subsequent illumination by the blue light during CW-OSL readout. This also indicates that all the TL peaks are contributing in OSL one way or another. Finally, TL glow curves were recorded after each subsequent CW-OSL readouts a number of times (Fig. 10). It was surprising that there was no decrease in the TL intensity as compared to the subsequent OSL readouts. This might be occurring due to a lower probability of transitions of the deeper traps to the conduction band and the subsequent recombination. From the above discussion it seems that even after optical annealing or thermal cleaning a small fraction of the deep traps still remain inside the material, which could be responsible for the supralinearity of the dose response. The amount of these reminiscent traps may depend upon the total number of traps created during irradiation (dose). This amount would be negligibly small for low doses and will not be that prominent, but at higher doses the effect is prominent until saturation occurs. | ||
| Fig. 10 TL glow curves recorded after successive CW-OSL decay curve readouts. The samples were annealed at 873 K for 1 h and irradiated by 1.0 Gy of γ rays from 137Cs source. | ||
Comparison of the OSL sensitivity of Na2LiPO4:Eu with those of Al2O3:C and BeO dosimeters
The CW-OSL intensity of the newly developed homemade Na2LiPO4:Eu OSLD phosphor was compared with that of commercially available Al2O3:C OSL dosimeters (Landauer, USA) and that of the BeO hot pressed chips (Thermalox® 995, Materion, USA). The results are as shown in Fig. 11. It can be seen from the figure that the intensity of our phosphor was found to be approximately 3 times less than that of the commercially available phosphors in the linear dose response range (i.e. 0.05–10.0 Gy). However, the advantage of our OSLD phosphor is the very easily available and inexpensive ingredients and a very simple method of preparation that make it cost effective as compared to the commercially available ones. Also, there is ease of handling, unlike with the Thermalox® 995 (BeO) dosimeters which are very much toxic.Fading
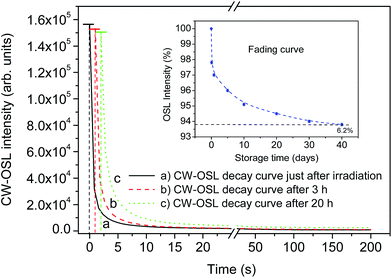
To study fading, the samples were irradiated by 1.0 Gy of γ rays from a 137Cs source. The samples were stored at room temperature (∼300 K) in the dark and CW-OSL was taken at different intervals of time. The results are as shown in Fig. 11 (inset). A few CW-OSL decay curves recorded initially and after 3 h and 20 h are also given. Other decay curves are not given for clarity of the figure. It can be seen from Fig. 12 that ∼6.2% fading of the OSL signal was observed in 40 days. This is very much comparable with that of the commercially available phosphors, i.e., Al2O3:C (Luxel™, Landauer Inc., USA) and BeO (Thermalox® 995, Materion Inc., USA). This has also been accepted for medical and personnel dosimetry.Optical annealing (bleaching) and reusability
Several batches of the newly developed NaLi2PO4:Eu OSLD phosphor were prepared and used for OSL studies. The phosphors were also irradiated to various test doses; OSL was taken and optically annealed using an annealing system consisting of 8 high output T5 fluorescent lamps (Annealing System, Model no.: GS-00004, Gammasonics, Australia). The system can remove a dose of a few mGy in a few minutes.31 The samples were optically annealed for different times after the OSL readouts until the entire OSL signal was erased. After the first CW-OSL, the remaining signal is of the order of only a few mGy. The moderate time for annealing/bleaching was found to be around half an hour. The OSL was again taken to see that no OSL signal was left inside the material. Several samples were irradiated at different test doses and the procedure was repeated several times. A few results are shown in Fig. 13. The material shows excellent reusability.Theoretical fitting of the CW-OSL decay curve and photo-ionization cross-sections
Fig. 14 and 15 show theoretical curve fittings of typical CW-OSL decay curves for the OSL stimulated by the blue (470 nm) and the green (530 nm) light. The figure of merit (FOM) for both the curve fittings was approx. 2%. It can be observed from the figure that the blue light stimulated decay curve consists of two components, a fast component and a slow component, while that stimulated by the green light consists of three components, fast, medium and slow. The sum of these components is also shown as theoretically fitted curves (curves with symbols). The curve stimulated by blue light can, be represented by the following equation (eqn (1)).
 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 734.5, τ1 = 3.45, A2 = 4
734.5, τ1 = 3.45, A2 = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440
440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 293.7, τ2 = 0.50 and the other one (stimulated by green light) by another equation as given below (eqn (2)),
293.7, τ2 = 0.50 and the other one (stimulated by green light) by another equation as given below (eqn (2)),
 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 656.8, τ1 = 1.14, A2 = 107
656.8, τ1 = 1.14, A2 = 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 279.3, τ2 = 0.10, A3 = 34
279.3, τ2 = 0.10, A3 = 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103.1, τ3 = 11.7.
103.1, τ3 = 11.7.
From the above discussion, it is clear that the decay curve stimulated by green light is more complicated than that stimulated by blue light. Therefore, stimulation by blue light is preferred. It may also be inferred that different kinds of traps might have been involved in the two different stimulation processes.
The photo-ionization cross-section was determined by using the following formula for photoionization cross-section, σ = 1/(ϕ × τ), where τ is the decay constant (in seconds) and ϕ is the incident photon flux (photons incident per cm2 per second). The corresponding values of decay constants and photo-ionization cross-sections are as given in Table 1. It can be seen from this table that the photoionization cross-sections depend not only on the stimulating wavelength (energy) but also on the total incident photon flux at the sample. In the present case, large differences in the photo-ionization cross-sections may also be attributed to different incident photon fluxes due to the different powers of the stimulating sources.
Experimental
Material and the method
Eu3+-doped orthophosphate NaLi2PO4 phosphor was synthesized by a simple solid-state diffusion method.20 This is described here briefly. The starting materials LiOH, NaH2PO4 (CDH, 99.5%) and the impurity salt EuCl3 (Alfa Aesar, 99.9%) were used to synthesize the material. All the chemicals were used as received without any further purification as they were of high purity. The samples were prepared by considering the following chemical reaction:The impurity (in appropriate amounts, i.e., 0.05–3.0 mol%) was firstly dissolved in LiOH water solution and the solvent was evaporated slowly in a vacuum oven. The Eu-doped LiOH (thus obtained) and NaH2(PO)4 (in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) were mixed thoroughly, using an agate mortar and pestle, in the presence of ethanol for better mixing. A temperature-controlled programmable furnace having a temperature stability better than ±1 K was used for synthesis of the material. The mixture was heated initially at 673 K for 12 h in air and then cooled slowly to room temperature, crushed again to a fine powder and reheated at 1073 K for the same period. The ingots thus obtained were finally crushed and sieved to get particle sizes in the range of 100–200 μm, given proper heat treatment and were used for further characterizations.
1 molar ratio) were mixed thoroughly, using an agate mortar and pestle, in the presence of ethanol for better mixing. A temperature-controlled programmable furnace having a temperature stability better than ±1 K was used for synthesis of the material. The mixture was heated initially at 673 K for 12 h in air and then cooled slowly to room temperature, crushed again to a fine powder and reheated at 1073 K for the same period. The ingots thus obtained were finally crushed and sieved to get particle sizes in the range of 100–200 μm, given proper heat treatment and were used for further characterizations.
Characterization
The powder X-ray diffraction (XRD) patterns were recorded on a high-resolution D8 Discover Bruker X-ray diffractometer using a monochromatic Cu-Kα1 line obtained through a pair of Göbel mirrors. The scan rate was kept at 1.0 s per step with a step size of 0.02 s. The XRD recording was done at room temperature. The CW-OSL decay curves were recorded on a Risø TL/OSL Reader Model TL/OSL-DA-20TL (Risø, DTU, Radiation Research Division, Denmark) available at AIIMS, New Delhi, India. The decay curves were recorded for 300 s using blue (470 nm, FWHM ≈ 20 nm) and green (530 nm, FWHM ≈ 20 nm) LED lights (variable from 50 to 100 mW cm−2) at the sample position for stimulation and a wide band photomultiplier tube (EMI 9235QB15 PMT, ET Enterprises Ltd, UK) for the detection of light. A UV transmitting broad-band glass filter (Hoya U-340, 7.5 mm total thickness, transmission between 270 nm and 380 nm, Hoya Corporation, Japan) was used (across the PMT) to prevent a stimulation signal from reaching it and a long pass green filter GG-420 (3.0 mm thickness, Schott, Germany) was used (across the LED) to cut off the stimulation wavelengths below 420 nm. For low doses the samples were exposed in situ to the 90Sr–90Yr β rays source (dose rate 0.16 Gy min−1) attached to the main reader unit (∼3.0 mGy–140 mGy). However, for higher doses a 137Cs γ rays source (dose rate 0.6 Gy min−1) was used (1.0 Gy–1 kGy). Every time ∼10 mg preirradiated sample was used for the CW-OSL decay curve recordings. For comparative and other studies, some TL measurements were also carried out using the same TL/OSL set up. Recordings of the TL glow curves and CW-OSL decay curves after partial thermal cleaning were also done to study whether all the traps take part in the OSL. All the samples were protected from sun/room light during irradiation as well as during storage and measurements.Conclusions
The newly developed NaLi2PO4:Eu phosphor material shows good OSL characteristics useful for the dosimetry of high-energy radiations. The newly developed phosphor material was compared with the commercially available Al2O3:C (Luxel™, Landauer, USA) and BeO (Thermalox® 995, Materion, USA) phosphors. Though the phosphor is a little less sensitive (∼3 times less), the very easy method of synthesis makes it very cost effective. The fading in the material was found to be around 6.2% in 40 days and is comparable with that of the commercially available phosphors and thus is acceptable for dosimetry. The dose response was found to be linear in the dose range of 0.05–0.0 Gy but shows supralinearity at higher doses (from 10.0 to 100 Gy) before it gets saturated. The supralinearity and the contribution of different kinds of traps were also studied in detail and explained.Acknowledgements
We are thankful to the Inter-University Accelerator Center, New Delhi, for partial financial assistance under the research project (UFR # 45318) and also University of Delhi for the research grant under the R & D scheme (RC/2014/6820). We are also thankful to the Director, BRAIRCH, AIIMS, New Delhi for permitting us to use the facilities at the hospital. We are also thankful to Mrs Debbie Handley from Materion, USA, for generously donating a few BeO (Thermalox® 995) dosimeter chips.References
- L. Botter-Jensen, S. W. S. McKeever and A. G. Wintle, Optically Stimulated Luminescence Dosimetry, Elsevier Science B. V., Amsterdam, The Netherlands, 2003 Search PubMed.
- M. Danilkin, A. Lust, A. Ratas, V. Seeman and M. Kerikmäe, Radiat. Meas., 2008, 43, 300 CrossRef CAS PubMed.
- G. G. Cai, J. Fesquet, L. Dusseau, M. Martini, F. Meinardi, B. L. Huang, K. Y. Tang, D. Beteille and J. Gasiot, Radiat. Prot. Dosim., 1996, 65, 163 CrossRef CAS.
- B. Yang, Q. Lu, S. Wang and P. D. Townsend, Nucl. Instrum. Methods Phys. Res., Sect. B, 2005, 239, 171 CrossRef CAS PubMed.
- M. Singh and P. D. Sahare, Nucl. Instrum. Methods Phys. Res., Sect. B, 2012, 289, 59 CrossRef CAS PubMed.
- M. S. Akselrod, V. S. Kortov, D. J. Kravetsky and V. I. Gotlib, Radiat. Prot. Dosim., 1990, 32, 15 CAS.
- M. S. Kulkarni, D. R. Mishra, K. P. Muthe, A. Singh, M. Roy, S. K. Gupta and S. Kannan, Radiat. Meas., 2005, 39, 277 CrossRef CAS PubMed.
- M. Sommer, R. Fraudenberg and J. Henniger, Radiat. Meas., 2007, 42, 617 CrossRef CAS PubMed.
- B. Dhabekar, S. N. Menon, E. Alagu Raja, A. K. Bakshi, A. K. Singh, M. P. Chougaonkar and Y. S. Mayya, Nucl. Instrum. Methods Phys. Res., Sect. B, 2011, 269, 1844 CrossRef CAS PubMed.
- R. A. Barve, R. R. Patil, S. V. Moharil, N. P. Gaikwad, B. C. Bhatt, R. Pradeep, D. R. Mishra and M. S. Kulkarni, Radiat. Prot. Dosim., 2014 DOI:10.1093/rpd/ncu255.
- A. Soni, K. P. Muthe, M. S. Kulkarni, D. R. Mishra, B. C. Bhatt, S. K. Gupta, J. V. Yakhmi and D. N. Sharma, J. Lumin., 2010, 130, 1308 CrossRef CAS PubMed.
- M. S. Kulkarni, K. P. Muthe, N. S. Rawat, D. R. Mishra, M. B. Kakade, S. Ramanathan, S. K. Gupta, B. C. Bhatt, J. V. Yakhmi and D. N. Sharma, Radiat. Meas., 2008, 43, 492 CrossRef CAS PubMed.
- R. Barve, R. R. Patil, N. S. Rawat, N. P. Gaikwad, R. Pradeep, B. C. Bhatt, S. V. Moharil and M. S. Kulkarni, Nucl. Instrum. Methods Phys. Res., Sect. B, 2012, 289, 100 CrossRef CAS PubMed.
- J. I. Lee, A. S. Pradhan, J. L. Kim, I. Chang, B. H. Kim and K. S. Chung, Radiat. Meas., 2012, 47, 837 CrossRef CAS PubMed.
- A. J. Bos, M. Prokic and J. C. Brouwer, Radiat. Prot. Dosim., 2006, 119, 130 CrossRef CAS PubMed.
- C. Dotzler, G. V. M. Williams, U. Reiser and A. Edgar, Appl. Phys. Lett., 2007, 91, 121910 CrossRef PubMed.
- S. W. S. McKeever, Optically Stimulated Luminescence Dosimetry, Nucl. Instrum. Methods Phys. Res., 2001, 184, 29 CrossRef CAS.
- M. S. Kulkarni, Internat. J. Lum. Appl., 2012, 2, 84 Search PubMed.
- A. S. Pradhan, J. I. Lee and J. L. Kim, J. Med. Phys., 2008, 33, 85 CrossRef CAS PubMed.
- P. D. Sahare and M. Singh, Indian J. Phys., 2014, 88, 621 CrossRef CAS.
- M. Singh, P. D. Sahare and P. Kumar, Radiat. Meas., 2013, 59, 8 CrossRef CAS PubMed.
- G. Y. Chao and T. S. Ercit, Can. Mineral., 1991, 29, 565 CAS.
- S.-S. Chang and M. S. Jo, Ceram. Int., 2007, 33, 511 CrossRef CAS PubMed.
- A. Lust, Ph. D. thesis, Charge state of dopants and ordered clusters formation in CaF2:Mn and CaF2:Eu luminophors, Tartu University Press, ISBN: 978-9949-11-6-621, 2007.
- P. D. Sahare and M. Singh, Indian J. Phys., 2014, 88, 621 CrossRef CAS.
- R. Nakata, K. Kohnom, M. Sumita and E. Higuchi, J. Phys. Soc. Jpn., 1976, 41, 470 CrossRef CAS.
- F. M. Lay and A. W. Nolle, Phys. Rev., 1967, 163, 266 CrossRef CAS.
- A. R. Lakshmanan, M. T. Jose, V. Ponnusamy and K. P. R. Vivek, J. Phys. D: Appl. Phys., 2002, 35, 386 CrossRef CAS.
- C. M. Sunta, Radiat. Prot. Dosim., 1984, 8, 25 CAS.
- N. Mandlik, P. D. Sahare, M. S. Kulkarni, B. C. Bhatt, V. N. Bhoraskar and S. D. Dhole, J. Lumin., 2014, 146, 128 CrossRef CAS PubMed.
- Webpage: http://portal.landauer.com.au/.
| This journal is © The Royal Society of Chemistry 2015 |