Nanoflake driven Mn2O3 microcubes modified with cooked rice derived carbon for improved electrochemical behavior†
B. Chandra Sekhara,
Ganguli Babub and
N. Kalaiselvi*a
aCSIR-Central Electrochemical Research Institute, Karaikudi-630 006, Tamilnadu, India. E-mail: kalaiselvicecri@gmail.com
bWayne State University-Department of Mechanical Engineering, Michigan 48202-3902, USA
First published on 5th December 2014
Abstract
Mn2O3 microcubes, symmetrically formed out of the systematic stacking of nanoflakes, built with nanoparticles in the 30–50 nm range have been obtained from a simple co-precipitation method. Excluding the requirement of a structure directing additive, the currently adopted synthesis protocol signifies the vital role of the rate of (NH4)HCO3 precursor addition, which has been optimized as 2 h for 300 mL to obtain uniformly stacked nanoflakes of Mn2O3 to form microcubes with desired morphological features. Cooked rice carbon (CRC), obtained from a filth-to-wealth conversion, has been used as conducting additive and an optimum concentration of 20 wt% CRC was found to be sufficient to form Mn2O3/CRC with improved lithium intercalation/de-intercalation behavior. The twin advantages, namely exploitation of a cheap and eco-benign composite additive obtained from a common domestic waste in the form of CRC and the optimized speed of addition of (NH4)HCO3 to form Mn2O3 microcubes obtained from nanoflakes offer advantages in terms of enhanced electronic conductivity and provision to buffer the volume changes of Mn2O3 anode, respectively. The optimized Mn2O3/CRC-20 composite anode exhibits an appreciable capacity of 830 mA h g−1 after formation cycle and an acceptable capacity of 490 mA h g−1 after completing 100 cycles, under the influence of 50 mA g−1 current density. Further, Mn2O3/CRC-20 anode exhibits a reasonable capacity of 450 mA h g−1 at 100 mA g−1 up to 50 cycles and qualifies itself as a potential anode material.
1. Introduction
Rechargeable lithium-ion batteries, bestowed with high voltage, capacity and appreciable cycle life are considered as one of the most promising electrochemical power sources, as they are widely used in many portable applications such as mobile phones, laptops and cameras. It is well known that a combination of all the desired properties in one electrode is generally not possible. However, one can fine tune lithium intercalating anodes possessing said properties by way of manipulating the physical properties, which in turn could be achieved by adopting suitably designed synthesis protocol. Approaches to construct structures such as mesoporous,1,2 hollow spheres,3,4 nanospheres,5 nanowires,6 nanoplatelets,7 nanorods,8 nanosheets,9 nanocages,10 nanotubes11 etc. are reported. Of the known structures, micro/nanocubes are rarely reported, due to the requirement of stringent synthesis conditions to obtain the final product in the form of regularly shaped cubes.Apart from the well-known advantages of nanostructured electrode materials, the associated disadvantages such as low density and high risk of surface side reactions could be addressed,12 especially when such electrode materials are prepared in the form of a composite consisting of micro/nano architecture. Herein, the micron sized particles offer advantages to offset the critical issues related to nanostructured electrode material. In addition, synergetic advantages of integral microstructure and short diffusion length of the nanoparticles could be achieved in the final product.
Considering the development of alternative anodes for graphite, transition metal oxides including MnO,13 CoO,14 Fe2O3,15 CuO,16 Cu2O,17 and NiO18 have been studied for application in lithium-ion batteries. Among the transition metal oxides, manganese oxide anodes (MnO, MnO2, Mn2O3 and Mn3O4) are of great interest for reasons such as natural abundance, economically viable and environmentally benign nature of manganese. Particularly, Mn2O3 assumes importance due to the high theoretical capacity (1018 mA h g−1) and lower operating voltage (discharge voltage is 0.5 V and charge voltage is 1.2 V) benefits.
Mn2O3 could be prepared in the form of spheres, cubes and plates by adopting methods such as solvothermal, precipitation and polyol solution respectively.19–21 Quite different from such approaches and from the recent reports on the synthesis of Mn2O3 cubes, requiring a structure directing group,22 the present study discusses on the possibility of adopting a simple co-precipitation method with a specific control on the speed of addition of (NH4)HCO3 precursor to prepare Mn2O3 cubes containing micro/nanostructured particles. These Mn2O3 micro/nanostructured cubes are subsequently modified as composites by incorporating an economically viable carbon, viz., cooked rice derived carbon (CRC) as the composite additive. Despite the number of reports available on the different types of carbon exploited for the said purpose, the study explores the possibility of exploiting carbon, derived from the wasted cooked rice as a compositing additive to improve the electrochemical behavior of pristine Mn2O3 cubes.
Rice, a popularly known carbohydrate, rich in carbon content has been made cheap, especially in India due to government policies. Besides edible purpose, rice starch finds its application in textile industry23 and acts as an energy efficient fuel24 to prepare wide variety of ceramics,25 semiconducting and electrochemically active electrode materials.26 However, rice being the major component of South Indian food item, especially after cooking, gets wasted in larger quantities from restaurants, hostels and community halls, apart from domestic wastage at home. Such a waste material, considered to be unfit for any other purpose has been collected and processed to obtain CRC through a filth-to-wealth attempt, that has further been deployed as a composite material to possibly improve the electrochemical performance of Mn2O3 cubes, obtained from a simple co-precipitation method without deploying any structure directing additive.
The present study is thus bestowed with twin novelty components, viz., exploration of CRC as a conducting additive to improve the lithium intercalating behavior of Mn2O3 microcubes (obtained from nanoflakes) and the optimisation of rate of addition of (NH4)HCO3 to obtain symmetrically formed Mn2O3 cubes. Because, there are reports available only on the effect of concentration of added sulphate (SO42−) precursor to prepare MnCO3 spheres27 and not on the effect of rate of addition of (NH4)HCO3 precursor to obtain MnCO3 cubes. Herein, we report for the first time about the change in the morphology of Mn2O3 final product, which could be tuned as a function of rate of addition of (NH4)HCO3 precursor. A shift from spheres – nano flakes (with desired thickness) – cubes (via stacking of nano flakes) has been noticed with a change in the rate of addition of (NH4)HCO3. Role of added CRC, rate of addition of (NH4)HCO3 precursor, effect of morphology and the optimization of CRC required to improve the electrochemical properties of Mn2O3 are discussed in this communication. Of the attempted combinations, Mn2O3/CRC-20 anode exhibits superior electrochemical performance, viz., 830 mA h g−1 as initial and 490 mA h g−1 as progressive capacity up to 100 cycles, resulting from the desirable combination of micro/nanostructure and the regularly formed cubes obtained from systematically stacked Mn2O3 nano flakes along with the added CRC.
2. Experimental section
2.1. Materials and synthesis
Mn2O3/CRC was prepared by mixing Mn2O3 cubes with CRC (10, 20 and 30% ratio), ground for 30 min and subsequently heated in the furnace at 400 °C for 2 h in Ar atmosphere to get better adherence. With a view to understand the role and to optimise the amount of CRC required to improve the electrochemical performance of pristine Mn2O3 microcubes, three different ratios of CRC, viz., 10, 20 and 30 wt% were chosen for the study. The composites obtained as the final product will be represented hereafter as Mn2O3/CRC-10, Mn2O3/CRC-20 and Mn2O3/CRC-30 respectively to represent the addition of 10, 20 and 30 wt% of cooked rice derived carbon to pristine Mn2O3 to form the final composite. Such composites were characterised individually to better understand the role of CRC and the extent of improvement observed in terms of electrochemical properties, as a function of added amount of CRC.
3. Physicochemical characterization
The crystal structure information of the synthesized compound was analysed with Bruker D8 Advance X-ray diffractometer using Ni-filtered Cu Kα radiation (λ = 1.5406 Å). Fourier transform infrared spectroscopy (FT-IR) studies were carried out using Bruker Tensor 27 FT-IR Spectrometer. By using a TA instrument SDT Q600 thermogravimetric analyser, TGA studies were carried out. To study the particle size, surface morphology and the presence of carbon coating, scanning electron microscopy (SEM, JEOL JSM6480LV system), field emission scanning electron microscopy (FESEM, Gemini) and transmission electron microscopy (TEM, Tecnai 20 G2 (FEI make)) were used. The chemical composition of the products has been obtained from X-ray photoelectron spectroscopy (XPS, MULTILAB 2000 Base system with X-ray, Auger and ISS attachments). The surface area and the pore size of the samples were determined by Brunauer–Emmett–Teller (BET) method by nitrogen adsorption/desorption using Quantachrome, NOVA version 11.02. VMP3 multichannel potentiostat–galvanostat system (Biologic Science Instrument) was used to study the cyclic voltammetry (CV) in the potential window of 0.01–3.0 V and at a scan rate of 0.1 mV s−1. Charge–discharge cycling studies were performed with Arbin cycler.4. Electrochemical characterization
The electrochemical measurements were carried out using CR2032 type coin cells, wherein copper foil has been used as the current collector and lithium metal as counter and reference electrode. A solution consisting of 1.0 M LiPF6 dissolved in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture of ethylene carbonate (EC)/dimethyl carbonate (DMC) serves as the electrolyte. The working electrode was fabricated by mixing Mn2O3 microcubes as the active material, conductive carbon (Super-P) and polyvinylidene fluoride binder in the weight ratio of 70
1 (v/v) mixture of ethylene carbonate (EC)/dimethyl carbonate (DMC) serves as the electrolyte. The working electrode was fabricated by mixing Mn2O3 microcubes as the active material, conductive carbon (Super-P) and polyvinylidene fluoride binder in the weight ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Using N-methyl-2-pyrrolidone as solvent, the mixture was made as slurry and coated on copper foil. The coated electrode was dried in oven at 80 °C to evaporate NMP, hot roll pressed to ensure better adherence and cut in to circular discs of 15.5 mm diameter to act as anode in the coin cell assembly.
10. Using N-methyl-2-pyrrolidone as solvent, the mixture was made as slurry and coated on copper foil. The coated electrode was dried in oven at 80 °C to evaporate NMP, hot roll pressed to ensure better adherence and cut in to circular discs of 15.5 mm diameter to act as anode in the coin cell assembly.
5. Results and discussion
5.1. Physical characterisation of Mn2O3 cubes obtained from MnCO3 nanoflakes and the formed Mn2O3/CRC composite
From Scheme 1, it is evident that the added (NH4)HCO3 with an optimum speed (300 mL/2 h) reacts with MnSO4 (a) to form MnCO3 flakes (b), which in turn stacks together in a symmetrical manner to form MnCO3 cubes (c) ((ESI), Fig. S1†). The cubes thus obtained release CO2 gas upon furnace heating at 600 °C for 2 h to produce Mn2O3 cubes (d) formed out of Mn2O3 nanoflakes. Further, Mn2O3/CRC composite is obtained when Mn2O3 cubes are heated with CRC at 400 °C for 2 h in Ar atmosphere (e).X-ray diffraction (XRD) measurements were carried to understand the structure and phase of the precursor and the final product obtained as per Scheme 1. Fig. 1 shows the XRD pattern of MnCO3, Mn2O3 and Mn2O3/CRC composites. Fig. 1a shows the diffraction peaks of MnCO3, wherein all the peaks could be indexed to the rhodochrosite structure with R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c space group, as per JCPDS card no. 86-0172. The lattice constant values are a = 4.78 and b = 15.61. No impurity peaks are observed and from this, one can understand that high purity MnCO3 powder has been formed out of this synthesis. Fig. 1b represents the XRD pattern of Mn2O3 powder, which shows striking similarity with the cubic Mn2O3 (JCPDS card no. 01-1061) product.
c space group, as per JCPDS card no. 86-0172. The lattice constant values are a = 4.78 and b = 15.61. No impurity peaks are observed and from this, one can understand that high purity MnCO3 powder has been formed out of this synthesis. Fig. 1b represents the XRD pattern of Mn2O3 powder, which shows striking similarity with the cubic Mn2O3 (JCPDS card no. 01-1061) product.
As there are no peaks corresponding to MnCO3 powder, it is understood that complete decomposition of MnCO3 has taken place to form phase pure Mn2O3. Fig. 1c–e corresponds to the XRD of Mn2O3/CRC composite products, wherein the presence of sharp and strong peaks confirms the crystallinity of the final product obtained. Among the three composites, Mn2O3/CRC-10 shows no change in the XRD pattern, peak position and intensity. However, Mn2O3/CRC-20 exhibits slightly broader Bragg peaks, thus indicating the nanocrystalline product formation, aided by the increased carbon content. On the other hand, Mn2O3/CRC-30 shows diminished (211) peak at 2θ = 23.7° and slightly enhanced intensity for peaks located at 2θ = 61.3° and 64.8°, which is considered as a deviation from the standard pattern. As a result, one can deduce the information that Mn2O3/CRC-20 provides interesting Bragg behavior, leaving behind some scope to consider the same as a better composition, compared with the rest of the composites.
Interestingly, the XRD pattern of CRC (ESI, Fig. S2a†) contains two broad peaks at 2θ ranging from 20 to 28° and at 2θ = 44°, corresponding to the (002) and (101) planes, thus representing the graphitic stacking pattern of carbon. Hence, it is presumed that CRC might possess high electrical conductivity. Further, Raman spectrum of CRC shows two peaks at 1350 and 1580 cm−1 corresponding to the defect (D) and graphitic band (G) (ESI, Fig. S2b†), which could be ascribed to the vibrations of carbon atoms with sp2 electronic configuration.28,29 The G band is found to be narrow and exhibits slightly higher intensity than the corresponding D band, thus confirming the possibility that CRC possesses low graphitization degree.30 In other words, ID/IG ratio is 0.86, which indicates that small amount of defects are present in the sample. Raman spectrum of Mn2O3/CRC-20 composite (ESI, Fig. S3†) consists of a high intensity peak at 658 and low intensity peaks at 580 and 478 cm−1, which is an indication of the formation of Mn2O3 possessing well defined electronic states.31,32
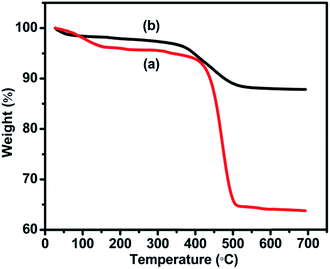
Fig. 2 depicts the TG analysis of the formed intermediate product viz., MnCO3 nanoflakes and Mn2O3/CRC-20 obtained as the final product. According to the TGA curve, it is evident that 405 °C is the decomposition temperature of MnCO3 to form Mn2O3. In Fig. 2a, two weight loss regions corresponding to 150 and 405 °C are seen. The weight loss around 150 °C is caused due to the removal of water from the sample. The subsequent and drastic weight loss around 405 °C is ascribed to the thermal decomposition of MnCO3 to form Mn2O3, by the evolution of CO2 and MnO.33,34 In Fig. 2b, the first weight loss at 150 °C is due to the removal of water/moisture from CRC and the second weight loss in the 370–530 °C region is due to the carbon from CRC present in the Mn2O3/CRC composite.
To further understand the local cation arrangement of MnCO3 and Mn2O3 microcubes, FT-IR was recorded and the results are spectacle in Fig. 3. MnCO3 shows peaks at 2480, 1398, 860 and 717 cm−1, which are attributed to the C–O bending vibration of carbonates (Fig. 3a).35 FT-IR signature recorded for Mn2O3 is displayed in Fig. 3b, wherein carbonate peaks of MnCO3 are not found and the observed peaks are matching with the title compound Mn2O3.36 The presence of three peaks around 514, 578 and 682 cm−1 may be attributed to Mn–O and hence the formation of MnCO3 and Mn2O3 could be understood.37
XPS is one of the best implements to identify the elemental composition and chemical bonding of the formed material. Fig. 4a and b represent the XPS survey spectra corresponding to those of Mn2O3 microcubes and Mn2O3/CRC composite, wherein four main peaks located at 284.7, 531.3, 641.9 and 653.7 eV, pertinent to elements such as C 1s, O 1s, Mn 2p3/2 and Mn 2p1/2 respectively are seen. Fig. 4c and d exhibit the deconvoluted XPS spectra of the Mn 2p, wherein two peaks located at 641.6 and 653.4 eV are attributed to Mn 2p3/2 and Mn 2p1/2, and the corresponding spin energy separation of 11.8 eV is matching with that of Mn2O3.38 O 1s spectrum shows four peaks after deconvolution and are positioned at 529.8, 530.9, 531.9 and 533.7 eV in Fig. 4e and f. Such peaks could be assigned to the presence of oxygen in the binary oxide.39–41 The peaks of C 1s are attributed to graphitized carbon and some O–C bonds are placed at 284.7, 285.8 and 288.4 eV.42 The recorded EDX spectrum confirms the presence of Mn and O in Mn2O3 and the presence of carbon along with Mn and O in the composite (ESI, Fig. S4†), thus substantiating the chemical composition and stoichiometry of the chosen Mn2O3 and its corresponding composite with CRC. The percentage composition of elements present in Mn2O3/CRC-20 clearly evidences the carbon content as 19.5, which is in agreement with the CRC-20 composite (ESI, inset of Fig. S4b†).
 | ||
| Fig. 4 XPS spectra of bare Mn2O3 and Mn2O3/CRC-20 composite; (a and b) survey spectrum, (c and d) Mn 2p, (e and f) O 1s and (g and h) C 1s spectrum. | ||
To study the morphology and particle size of MnCO3 (intermediate) and the final product, viz. Mn2O3, scanning electron microscopy (SEM) was recorded to capture the respective images. Typical images of the MnCO3 intermediate evidence the formation of perfect cubes with 2 μm size (ESI, Fig. S1†). Thermal decomposition of MnCO3 forms Mn2O3 and the morphological features (Fig. 5) correspond to the presence of cubes, obtained from the systematic stacking of nanoflakes. Fig. 5a shows low magnification images of Mn2O3, wherein more or less uniform size and shape of cubes are seen. Fig. 5b–d are high magnification images of Mn2O3, in which formation of symmetrical and individual cubes with an edge length of approximately 1.5 to 2.0 μm is evident. Systematic stacking of nanoflakes in a uniform manner with an inherent control (induced by the selected synthesis protocol) over the thickness and length spontaneously produces symmetrically formed Mn2O3 microcubes without any agglomeration, which is noteworthy. The shape and size of the Mn2O3 cubes are greatly influenced by the rate of addition of (NH4)HCO3 solution to the MnSO4·H2O precursor solution and by the concentration of SO42−.
The significant role of rate of addition of (NH4)HCO3 in deciding the morphology of (ESI, Fig. S5†) the final product in an indispensable manner could be understood from the following. The addition of (NH4)HCO3 solution (300 mL) in 30 min results in the formation of MnCO3 and Mn2O3 microspheres (ESI, Fig. S5a†), whereas an addition time of 2 h results in the formation of desired Mn2O3 microcubes containing properly stacked nanoflakes by allowing the existence of individual microcubes (ESI, Fig. S5b†). On the other hand, an addition time of 3 h leads to the formation of nanoflakes with increased thickness (55 nm), due to which, the Mn2O3 cubes have a tendency to undergo slight agglomeration (ESI, Fig. S5c†). The same is clearly evidenced in Fig. S5d† with highly agglomerated Mn2O3 particles obtained with the addition of (NH4)HCO3 in 4 h. As a result, coalesced cubes of Mn2O3 have been obtained, especially when the addition time exceeds 2 h. Hence, it is understood that the carefully chosen concentration (0.03 M) of (NH4)HCO3 needs to be added in an optimized time period of 2 h to obtain discrete cubes with desired thickness, edges and porosity.
Fig. 6 presents the FESEM images of the as-prepared pure Mn2O3 (a and b) and the composite of Mn2O3 obtained with 20% CRC powder (c and d). Fig. 6a represents the low magnification image of pure Mn2O3, wherein assembly of perfectly formed cubes from nanoparticles, possessing a size of around 30–50 nm, have been obtained, which is in agreement with the findings from transmission electron microscopy (TEM). In the high magnification image, it is clearly seen that a single Mn2O3 microcube contains symmetrically packed nanoflakes and the flakes are formed out of irregularly shaped nanoparticles that bridge with each other to realize a flake thickness of 35–45 nm, as seen in Fig. 6b. Fig. 6c and d are low and high magnification FESEM images of Mn2O3/CRC-20 composite respectively. Presence of carbon coating on Mn2O3 microcubes is obvious from the images, which are further substantiated by TEM results. Such a carbon coating is highly essential to provide better electronic conductivity through carbon wiring.
TEM images evidence the presence of cubes (Fig. 7a), formed out of nanoflakes (Fig. 7b) containing systematically grouped assembly of irregularly shaped nanoparticles of 30–50 nm size (Fig. 7c and d with a closer view). The presence of carbon coating (grey colour) found on the surface of individual Mn2O3 cubes is clearly seen in Fig. 7e and f. Existence of individual Mn2O3 cubes possessing a thin line of carbon coating to avoid agglomeration and to restrict/partially allow the unavoidable volume expansion is seen in the zoomed image of Fig. 7f (Fig. 7g). Similarly, presence of continuous chain like carbon network arrangement, otherwise known as carbon wiring of Mn2O3/CRC composite is evident from Fig. 7h. Closer view of TEM images of Mn2O3 nanoflakes is illustrated in Fig. S6(a–d),† which confirms the fact that the microcubes are built with the symmetrically formed nanoflakes. SAED pattern evidences the presence of irregular diffraction spots, corresponding to the presence of polycrystalline phase of bare Mn2O3 (inset of Fig. 7a). The surface area and porous structure of Mn2O3 and the corresponding Mn2O3/CRC-20 composite have been investigated by nitrogen isotherm adsorption–desorption measurement (ESI, Fig. S7†). The Brunauer–Emmett–Teller (BET) surface area of Mn2O3/CRC-20 composite is measured to be 67.9 m2 g−1, which is higher than that of Mn2O3 microcube (26.8 m2 g−1). The high surface area of the composite is associated with the presence of mesoporous particles, which in turn facilitates facile electrolyte accessibility, rapid lithium ion diffusion and buffering of volume expansion.43 Interestingly, Type-IV isotherm has been obtained, wherein the pore-size distribution for Mn2O3 microcube (46.2 nm) and Mn2O3/CRC-20 composite (38.1 nm) are shown in the inset of Fig. S7a and b,† indicating the presence of mesopores in the chosen products. Due to the presence of larger pores with open windows, CRC finds an easy access to the pores and forms a conducting carbon network in Mn2O3/CRC-20 composite, thus increasing the scope for improved performance of Mn2O3/CRC-20 electrode.44
5.2. Electrochemical characterization of pristine Mn2O3 and Mn2O3/CRC-20 composite anodes
| 2Li+ + 3Mn2O3 + 2e− → 2Mn3O4 + Li2O | (1) |
| 2Li+ + Mn3O4 + 2e− → 3MnO + Li2O | (2) |
| 2Li+ + MnO + 2e− → Mn + Li2O | (3) |
| Mn + xLi2O → 2xLi+ + MnOx + 2xe (1.0 < x < 1.5) | (4) |
 | ||
| Fig. 8 Cyclic voltammogram of (a) bare Mn2O3 and (b) Mn2O3/CRC-20 composite anode recorded at a scan rate of 0.1 mV s−1 in the range of 0.01–3.0 V versus Li+/Li. | ||
Upon comparison, the larger area and the increased peak current value exhibited by the cyclic voltammetry behavior of Mn2O3/CRC-20 anode indicates the beneficial effect of added CRC in improving the lithium intercalation/de-intercalation behavior with respect to pristine Mn2O3 anode.49–51 Since parameters such as nanoflakes and microcubes are commonly associated with both the anodes, the observed improvement in electrochemical properties is attributed to the advantageous effect of CRC in increasing the conductivity and facilitating the faster diffusion of lithium ions.
Fig. 9b displays the discharge and charge profile of Mn2O3/CRC-20 anode in the voltage range of 0.01 to 3.00 V with a current density of 50 mA g−1. The first discharge and charge capacity values are 2380 and 1084 mA h g−1 respectively, wherein a small portion of discharge capacity is obtained during the continuous decrease in the open circuit potential up to 1.35 V, which could be related to the reaction of Mn2O3 to form Mn3O4, as mentioned in eqn (1). The voltage then decreases to ∼0.34 V with the appearance of a new plateau. This may be attributed to the reduction of Mn3O4 to MnO, as expressed in eqn (2). The voltage further decreases gradually down to a deep discharge limit of 0.01 V, when the phase transformation reaction is taking place with the complete reduction of MnO to Mn and formation of Li2O, as per eqn (3) (inset of Fig. 9b). It is to be mentioned here that the first total discharge capacity is 2380 mA h g−1, which is twice the theoretical capacity of Mn2O3 (1018 mA h g−1). Probable reason for the realization of excess capacity could be related to the decomposition of the electrolyte at a lower voltage and the generation of a solid electrolyte interface (SEI) layer, wherein lithium storage takes place by interfacial charging at the metal/Li2O interface.37,52–54 Upon careful investigation of the first charge profile, a curve found at the voltage range from 1.0 to 1.4 V is associated with the oxidation of Mn to MnO. Subsequently, MnO reacts with Li2O, when the electrode potential exceeds 2.1 V and forms a mixture of MnO and MnOx (1.0 < X < 1.5) expressed by eqn (4). Based on this mechanism, Mn2O3/CRC-20 anode exhibits an appreciable second cycle capacity of 831 mA h g−1, (Fig. 9b) 50th cycle capacity of 505 mA h g−1 and a progressive steady-state capacity of 490 mA h g−1 at 50 mA g−1, even after completing 100 cycles. The corresponding coulombic efficiency behavior also increases from 43.5 to 99.9%, which is noteworthy. Similarly the cycling performance of Mn2O3/CRC-20 anode under a current density of 100 mA g−1 is also encouraging, wherein an appreciable capacity of 450 mA h g−1 has been obtained even after 50 cycles, which is higher than the reported results.55
Electrochemical impedance spectroscopy (EIS) was performed to understand the effect of added CRC in improving the conductivity as well as the lithium transport kinetics of Mn2O3/CRC composites (in comparison with that of pristine Mn2O3 anode) as a function of concentration of carbon. Electrochemical impedance behavior of the cells was measured in the frequency range of 10 kHz to 100 mHz. Fig. 10 shows the comparison of impedance behavior of bare Mn2O3 and Mn2O3/CRC composites measured at the open circuit voltage of the as fabricated cell. From the inset of Fig. 10, it is evident that the high frequency semicircle, attributed to the solid electrolyte interface film formation and/or contact resistance (Rs) corresponding to that of Mn2O3 anode exhibits high contact resistance compared with the composites viz., Mn2O3/CRC-10, 20 and 30 individually. The middle frequency of the semicircle due to the charge transfer resistance on the electrode and electrolyte interface (Rct), of Mn2O3/CRC-20 composite is found to be lower than the remaining composites and that of pristine Mn2O3 (Table 1). This could be understood in terms of the increased contact area benefit, resulting from the presence of nanocrystalline particles and the enhanced electrical conductivity of Mn2O3/CRC-20 electrode. At the low frequency region, corresponding to the diffusion of lithium ions within the electrode material, more or less straight line behavior is observed for Mn2O3/CRC-20 composite, compared with the remaining anodes, thus evidencing the faster diffusion of lithium-ions, as understood from the charge–discharge studies also. As mentioned already, the absence of carbon (pristine Mn2O3), lesser carbon with ineffective carbon wiring (Mn2O3/CRC-10) and higher carbon content than the optimum amount that impedes the facile diffusion of lithium ions (Mn2O3/CRC-30) are responsible individually for the currently observed higher internal resistance values, which are ultimately responsible for the inferior electrochemical properties compared with that of Mn2O3/CRC-20 anode.44,56–59
 | ||
| Fig. 10 Electrochemical impedance spectroscopy behavior of cells containing pristine Mn2O3 anode and Mn2O3/CRC anodes consisting of 10, 20 and 30 wt% CRC. | ||
| Anode | Rs (Ω) | Rct (Ω) |
|---|---|---|
| Pristine Mn2O3 | 23.6 | 39.5 |
| Mn2O3/CRC-10 | 10.8 | 35.4 |
| Mn2O3/CRC-20 | 10.8 | 32.6 |
| Mn2O3/CRC-30 | 13.5 | 38.3 |
6. Conclusion
Mesoporous Mn2O3 microcubes containing stacked nanoflakes composed of nanoparticles with irregular shape have been prepared without adding any surfactant. In order to impart value addition to the currently prepared Mn2O3 microcubes, a cheaper carbon additive, obtained from wasted cooked rice has been used to prepare Mn2O3/CRC composite. Optimized rate of addition of (NH4)HCO3 (300 mL/2 h), deployment of optimum concentration of CRC (20 wt%) and the presence of micro/nanostructure to admit the anticipated volume expansion and enhanced lithium diffusion kinetics in synergy have offered facile transportation of lithium ions and to recommend Mn2O3/CRC-20 as a better performing anode. An initial capacity of 830 mA h g−1 and a reasonable capacity of 490 mA h g−1 up to 100 cycles have been exhibited by Mn2O3/CRC-20 anode, when discharged at 50 mA g−1. The anode finds its suitability at various fairly high current densities also. The exploitation of symmetric Mn2O3 cubes for lithium battery application fetches interest to the composite and the synthesis protocol, apart from the application point of view.Acknowledgements
Financial support from University Grants Commission for the UGC-Senior Research Fellowship and Council of Scientific and Industrial Research (CSIR) through MULTIFUN program is gratefully acknowledged.References
- Y. F. Deng, Z. N. Li, Z. C. Shi, H. Xu, F. Peng and G. H. Chen, RSC Adv., 2012, 2, 4645–4647 RSC.
- X. M. Yin, L. B. Chen, C. C. Li, Q. Y. Hao, S. Liu, Q. H. Li, E. D. Zhang and T. H. Wang, Electrochim. Acta, 2011, 56, 2358–2363 CrossRef CAS PubMed.
- J. S. Chen, L. A. Archer and X. W. Lou, J. Mater. Chem., 2011, 21, 9912–9924 RSC.
- F. Zhang, Y. Zhang, S. Y. Song and H. J. Zhang, J. Power Sources, 2011, 196, 8618–8624 CrossRef CAS PubMed.
- Y. Sun, X. Y. Feng and C. H. Chen, J. Power Sources, 2011, 196, 784–787 CrossRef CAS PubMed.
- K. T. Nam, D. W. Kim, P. J. Yoo, C. Y. Chiang, N. Meethong, P. T. Hammond, Y. M. Chiang and A. M. Belcher, Science, 2006, 312, 885–888 CrossRef CAS PubMed.
- Y. Lu, Y. Wang, Y. Q. Zou, Z. Jiao, B. Zhao, Y. Q. He and M. H. Wu, Electrochem. Commun., 2010, 12, 101–105 CrossRef CAS PubMed.
- S. Vijayanand, R. Kannan, H. S. Potdar, V. K. Pillai and P. A. Joy, J. Appl. Electrochem., 2013, 43, 995–1003 CrossRef CAS.
- L. Pan, L. Li and Q. Y. Zhu, J. Sol-Gel Sci. Technol., 2013, 67, 573–579 CrossRef CAS PubMed.
- D. Q. Liu, X. Wang, X. B. Wang, W. Tian, Y. Bando and D. Golberg, Sci. Rep., 2013, 3, 2543–2548 Search PubMed.
- J. Xu, L. Gao, J. Y. Cao, W. C. Wang and Z. D. Chen, Electrochim. Acta, 2010, 56, 732–736 CrossRef CAS PubMed.
- A. K. Rai, J. Gim, L. T. Anh and J. Kim, Electrochim. Acta, 2013, 100, 63–71 CrossRef CAS PubMed.
- G. L. Xu, Y. F. Xu, H. Sun, F. Fu, X. M. Zheng, L. Huang, J. T. Li, S. H. Yang and S. G. Sun, Chem. Commun., 2012, 48, 8502–8504 RSC.
- M. Zhang, M. Q. Jia, Y. H. Jin and X. R. Shi, Appl. Surf. Sci., 2012, 263, 573–578 CrossRef CAS PubMed.
- J. Zhu, Z. Yin, D. Yang, T. Sun, H. Yu, H. E. Hoster, H. H. Hng, H. Zhang and Q. Yan, Energy Environ. Sci., 2013, 6, 987–993 CAS.
- L. L. Wang, H. X. Gong, C. H. Wang, D. Wang, K. B. Tang and Y. T. Qian, Nanoscale, 2012, 4, 6850–6855 RSC.
- D. Q. Liu, Z. B. Yang, P. Wang, F. Li, D. S. Wang and D. Y. He, Nanoscale, 2013, 5, 1917–1921 RSC.
- G. H. Zhang, Y. J. Chen, B. H. Qu, L. L. Hu, L. Mei, D. N. Lei, Q. Li, L. B. Chen, Q. H. Li and T. H. Wang, Electrochim. Acta, 2012, 80, 140–147 CrossRef CAS PubMed.
- L. Hu, Y. K. Sun, F. P. Zhang and Q. W. Xhen, J. Alloys Compd., 2013, 576, 86–92 CrossRef CAS PubMed.
- J. Cao, Y. c. Zhu, K. Y. Bao, L. Shi, S. Z. Liu and Y. T. Qian, J. Phys. Chem. C, 2009, 113, 17755–17760 CAS.
- Y. Zhang, Y. Yan, X. Wang, G. Li, D. Deng, L. Jiang, C. Shu and C. Wang, Chem.–Eur. J., 2014, 20, 6126–6130 CrossRef CAS PubMed.
- H. W. Shim, A. H. Lim, K. M. Min and D. W. Kim, CrystEngComm, 2011, 13, 6747–6752 RSC.
- J. A. Radley, Industrial Uses of Starch and its Derivatives, 1976, ISBN: 978-94-010-1331-4 Search PubMed.
- H. B. Goyal, D. Seal and R. C. Saxena, Renewable Sustainable Energy Rev., 2008, 12, 504–517 CrossRef CAS PubMed.
- E. Gregorova, W. Pabst and I. Bohacenko, J. Eur. Ceram. Soc., 2006, 26, 1301–1309 CrossRef CAS PubMed.
- Q.-Y. Li, H.-Q. Wang, Q.-F. Dai, J.-H. Yang and Y.-L. Zhong, Solid State Ionics, 2008, 179, 269–273 CrossRef CAS PubMed.
- J. B. Fei, Y. Cui, X. H. Yan, W. Qi, Y. Yang, K. W. Wang, Q. He and J. B. Li, Adv. Mater., 2008, 20, 452–456 CrossRef.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401–187404 CrossRef CAS.
- A. Maleservic, R. Vitchev, K. Schouteden, A. Volodin, L. Zhang, G. V. Tendeloo, A. Vanhulsel and C. V. Haesendonck, Nanotechnology, 2008, 19, 305604–305609 CrossRef PubMed.
- F. Zhang, K.-X. Wang, G.-D. Li and J.-S. Chen, Electrochem. Commun., 2009, 11, 130–133 CrossRef CAS PubMed.
- M. C. Bernard, A. H. L. Goff, B. V. Thi and S. C. Detorresi, J. Electrochem. Soc., 1993, 140, 3065–3070 CrossRef CAS PubMed.
- Q. Javed, F. P. Wang, M. Y. Rafique, A. M. Toufiq, Q. S. Li, H. Mahmood and W. Khan, Nanotechnology, 2012, 23, 415603–415610 CrossRef CAS PubMed.
- Y. Qiao, Y. Yu, Y. Jin, Y.-B. Guan and C.-H. Chen, Electrochim. Acta, 2014, 132, 323–331 CrossRef CAS PubMed.
- Y. F. Han, L. W. Chen, K. Ramesh, Z. Y. Zhong, F. X. Chen, J. H. Chin and H. W. Mook, Catal. Today, 2008, 131, 35–41 CrossRef CAS PubMed.
- H. Hu, j-y. Xu, H. Yang, J. Liang, S. Yang and H. Wu, Mater. Res. Bull., 2011, 46, 1908–1915 CrossRef CAS PubMed.
- X. Wang, S. Qiu, G. Lu, C. He, J. Liu, L. Luan and W. Liu, CrystEngComm, 2014, 16, 1802–1809 RSC.
- Y. Cai, S. Liu, X. M. Yin, Q. Y. Hao, M. Zhang and T. H. Wang, Phys. E, 2010, 43, 70–75 CrossRef CAS PubMed.
- A. A. Audi, P. M. Sherwood and A. Surf, Surf. Interface Anal., 2002, 33, 274–284 CrossRef CAS.
- A. Sakunthala, M. V. Reddy, S. Selvasekarapandian, B. V. R. Chowdari and P. C. Selvin, Energy Environ. Sci., 2011, 4, 1712–1725 CAS.
- J. Li, S. Xiong, X. Li and Y. Qian, J. Mater. Chem., 2012, 22, 23254–23259 RSC.
- Q. Li, L. Yin, Z. Li, X. Wang, Q. Yongxin and J. Ma, ACS Appl. Mater. Interfaces, 2013, 5, 10975–10984 CAS.
- J.-H. Zhou, Z.-J. Sui, J. Zhu, P. Li, D. Chen, Y.-C. Dai and W.-K. Yuan, Carbon, 2007, 45, 785–796 CrossRef CAS PubMed.
- F. Han, L.-J. Ma, Q. Sun, Ch. Lei and A.-H. Lu, Nano Res., 2014, 7, 1706–1717 CrossRef CAS.
- Xi. Fan, J. Shao, Xu. Xiao, L. Chen, X. Wang, S. Li and H. Ge, J. Mater. Chem. A, 2014, 2, 14641–14648 CAS.
- L. Chang, L. Mai, X. Xu, Q. An, Y. Zhao, D. Wang and X. Feng, RSC Adv., 2013, 3, 1947–1952 RSC.
- Y. F. Deng, Z. E. Li, Z. C. Shi, H. Xu, F. Peng and G. H. Chen, RSC Adv., 2012, 2, 4645–4647 RSC.
- Y. C. Qiu, G. L. Xu, K. Y. Yan, H. Sun, J. W. Xiao, S. H. Yang, S. G. Sun, L. M. Jin and H. Deng, J. Mater. Chem., 2011, 21, 6346–6353 RSC.
- L. Hu, Y. K. Sun, F. P. Zhang and Q. W. Xhen, J. Alloys Compd., 2013, 576, 86–92 CrossRef CAS PubMed.
- A. B. Yuan, S. A. Cheng, J. Q. Zhang and C. N. Cao, J. Power Sources, 1999, 77, 178–182 CrossRef CAS.
- L. Mai, X. Xu, C. Han, Y. Luo, L. Xu, Y. A. Wu and Y. Zhao, Nano Lett., 2011, 12, 4992–4996 CrossRef PubMed.
- X. Xu, Y. Z. Luo, L. Q. Mai, Y. L. Zhao, Q. Y. An, L. Xu, F. Hu, L. Zhang and Q. J. Zhang, NPG Asia Mater., 2012, 4, e20 CrossRef.
- X. F. Fang, X. Lu, X. W. Guo, Y. Mao, Y. S. Hu, J. Z. Wang, Z. X. Wang, F. Wu, H. K. Liu and L. Q. Chen, Electrochem. Commun., 2010, 12, 1520–1523 CrossRef CAS PubMed.
- S. Nayak, S. Malik, S. Indris, J. Reedijk and A. K. Powell, Chem.–Eur. J., 2010, 16, 1158–1162 CrossRef CAS PubMed.
- F. L. Wang, J. R. Liu, J. Kong, Z. J. Zhang, X. Z. Wang, M. Itoh and K. I. Machida, J. Mater. Chem., 2011, 21, 4314–4320 RSC.
- M.-W. Xu, Y.-B. Niu, S.-J. Bao and C. M. Li, J. Mater. Chem. A, 2014, 2, 3749–3755 CAS.
- S. Yang, H. Song and X. Chen, Electrochem. Commun., 2006, 8, 137–142 CrossRef CAS PubMed.
- A. Leela Mohana Reddy and S. Ramaprabhu, J. Phys. Chem. C, 2007, 111, 7727–7734 Search PubMed.
- B. Jin, E. M. Jin, K.-H. Park and H.-B. Gu, Electrochem. Commun., 2008, 10, 1537–1540 CrossRef CAS PubMed.
- L. Zeng, F. Xiao, J. Wang, S. Gao, X. Ding and M. Wei, J. Mater. Chem., 2012, 22, 14284–14288 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1: SEM images of MnCO3 microcubes with nanoflakes, S2: XRD and Raman behavior of cooked rice carbon (CRC), S3: complete Raman spectrum of Mn2O3/CRC-20, S4: EDX results of the pristine Mn2O3 and Mn2O3/CRC-20 composite, Fig. S5 SEM images of Mn2O3 obtained by varying the rate of addition of (NH4)HCO3 precursor, (a) spheres obtained in 30 min, (b) cubes obtained 2 h, (c and d) agglomerated cubes obtained 3 h and 4 h, S6: closer view of TEM images of Mn2O3 nanoflakes, S7: N2 adsorption–desorption isotherm of (a) Mn2O3 and (b) Mn2O3/CRC-20 composite. See DOI: 10.1039/c4ra11443d |
| This journal is © The Royal Society of Chemistry 2015 |