Graphene oxide coated quartz sand as a high performance adsorption material in the application of water treatment
Wenjun Houab,
Yimei Zhang*ab,
Tong Liuc,
Hongwei Lud and
Li Heabc
aSuzhou Research Academy of North China Electric Power University, Suzhou, Jiangsu, China. E-mail: Yimei.zhang1@gmail.com; Fax: +86-0512-67332655; Tel: +86-0512-67332668
bEnvironmental Research Academy of North China Electric Power University, Beijing, China
cSchool of water resource and electric power, Qinghai University, Xining, Qinghai 810016, People's Republic of China
dCollege of Renewable Energy, North China Electric Power University, Beijing 102206, China
First published on 19th December 2014
Abstract
In order to increase the water treatment performance, the quartz sand filter medium was improved by surface modification using a 3-aminopropyltriethoxy silane coupling agent (KH550), then GO was grafted to the surface of the sand though the chemical reaction between the functional groups. The interfacial interactions between the quartz sand surface and GO was studied. Fourier-transform infrared (FTIR) spectroscopy and X-ray photoelectron spectroscopy (XPS) analyses showed that the GO binds strongly to the quartz sand surface. Scanning electronic microscopy (SEM) observation showed that a thin GO layer was formed on the surface of the modified quartz sand. The modified sand was used as a sorbent for the removal of turbidity, organic matter, Cd(II) and Pb(II) ions from large volumes of aqueous solutions. The results indicate that the GO plays an important role in improving the water treatment performance of a quartz sand filter.
1. Introduction
Pollution of freshwater systems by industrial and agricultural waste has become one of the key environmental problems.1 Among all water contaminations, organic matter and heavy metals ions can cause severe health problems in humans and animals.2 For example, excessive Pb2+ could lead to a wide range of health problems, such as nausea, convulsions, coma, renal failure, cancer, and negative effects on metabolism and intelligence.3,4 Excessive Cd2+ could also cause a range of diseases, including hemochromatosis, gastrointestinal catarrh, cramps in the calves, skin dermatitis brass chills5 and Wilson disease.6 Many methods or technologies such as chemical precipitation, membrane processes, ion exchange, electrolysis, adsorption processes have been developed to remove the contaminations from wastewater.7–11 However, each method has been found to be limited by cost, complexity, efficiency as well as by secondary waste.2 Among these technologies, adsorptive removal of organic matter and heavy metals is widely applied because this method is easy to handle, relatively low cost, and effective at low concentration.12 Especially, the developments of nanoscience and biological science for organic matter and heavy metal removal have received remarkable attention due to their special features.13Following the discoveries of fullerene and carbon nanotubes (CNTs) in earlier decades, the emergence of graphene with its combination of extraordinary physical properties has opened up an exciting new field in the science and technology of two dimensional (2D) nanomaterials.14 Graphene's unique structural elements, including being a single two-dimensional and extensively conjugated, endow it with advantageous thermal conductivity,15 unique electric16 and mechanical properties,17 excellent mobility of charge carriers.18 Research has revealed that graphene has exhibited better adsorption behavior for heavy metal ions and fluoride than nanotubes.19,20 Unlike carbon nanotubes, which require special oxidation processes to introduce hydrophilic groups to improve metal ion sorption, the preparation of graphene oxide (GO) nanosheets from graphite using Hummers method introduces many oxygen-containing functional groups such as –COOH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and –OH, on the surfaces of GO nanosheets. These functional groups are essential for the high sorption of heavy metal ions.
O, and –OH, on the surfaces of GO nanosheets. These functional groups are essential for the high sorption of heavy metal ions.
Natural quartz sand (NS) is widely used for processes of the magnitude of municipal water supplies to small domestic water filters, particularly as packed bed filters. History, affordability and the granular nature of sand that forms filter beds have popularized sand-filtration (SF). Indeed, early Indian and Greek writings dating back 6000 years refer to sand and gravel-filtration as means to securing clean water and currently are a water purification process endorsed by the World Health Organization. Of the two broad classifications of SF, fine-SF has higher retention of pathogens, organic matter, and heavy metal ions but has low throughput. Although the production rates are higher for the more popular coarse-SF, the absence of functionality and nanostructures limit pathogen, organics and heavy metal ions retention.21–23
In Zhao's study,24 few-layered GO nanosheets were synthesized from graphite using the modified Hummers method, and were used as sorbents for the removal of Cd(II) and Co(II) ions from large volumes of aqueous solutions. The results indicated that abundant oxygen-containing functional groups on the surfaces of GO nanosheets played an important role on Cd(II) and Co(II) sorption. Gao25 demonstrated a simple technique for conversion of regular filtration sand into “core–shell” GO coated sand (GO–sand) granules by assembling water dispersible graphite oxide on sand grains. The results showed that the nanostructured GO-coated sand retains at least 5 fold higher concentrations of heavy metal and organic dye than pure sand. In this study, assembly process essentially consists of physical mixing of the water dispersible GO colloids with sand, followed by a mild heat treatment that causes the nanosheets to adhere to each other over the sand surface, likely through van der Waals interaction. Due to the combination of graphene and sand is simply the physical effect, so in the process of water treatment, graphene is easy to fell off the sand cause secondary pollution.
In order to solve the problem mentioned above, the surface modification of natural quartz sand was made by using 3-aminopropyltriethoxy silane coupling agent (KH550), then the surface functional groups of quartz sand reacted with modified GO, the color has gone from white to black (Fig. 1). So GO firmly planted on the surface of quartz sand, will not fall off and have secondary pollution. Through a series of experiments show that the GO coated sand (GOS) granules has a strong adsorption performance for organic matter and heavy metal ions.
2. Experimental
2.1 Materials
Graphite powder (20 mm), concentrated H2SO4, SOCl2, KMnO4, N,N-dimethylformamide (DMF), pyridine, 3-aminopropyltriethoxy silane coupling agent (KH550) and natural quartz sand were purchased from Sinopharm Chemical Reagent Co., Ltd.2.2 Synthesis of acyl chloride-functionalized GO (GO–Cl)
GO was fabricated from graphite powder (GP) by oxidation with KMnO4 in concentrated H2SO4, followed by hydrolysis, washing and centrifugation, according to the modified Hummers' method.26,27 This procedure has been confirmed by AFM to yield GO sheets with majority of monolayer dispersed structures.GO–Cl was prepared by reacting GO with SOCl2.28 As shown in Fig. 2, 50 mg GO was first sonicated in 20 mL of anhydrous DMF to form a homogeneous suspension, to which 50 mL SOCl2 was added dropwise at 0 °C. GO–Cl was prepared by stirring at 0 °C for 2 h, and keeping stirring for 36 h at 70 °C. The obtained GO–Cl was collected by filtration through a PTFE membrane.
2.3 Preparation of quartz sand filter
The quartz sand filter with size fractions of 0.55 to 0.83 mm was obtained by dry-sieving through stainless steel sieves. The filter medium was boiled in deionized water for 30 min followed by washing several times with deionized water until the washed water no longer seemed turbid. Before being used, the cleaned filter medium was dried at 110 °C for 12 h. This procedure substantially eliminates the effect of any impurity on the filter medium surface.2.4 Preparation of KH550-coated sand (KHS)
Synthesis route of KHS is show in Fig. 3. Firstly, 15% KH550 was added to alcohol–water solution to hydrolyze it sufficiently. The volume proportion of alcohol and water was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Secondly, the prepared quartz sand was added into above solution. The system was stirred and heated up to 70 °C using a water bath for 15 min followed by dried for 2.5 h at 110 °C. Thirdly, the reaction product was immersed in deionized water for 24 h and washed several times with deionized water to eliminate the influence of physical adsorption. Lastly, the surface modified filter was obtained by dried at 110 °C for 12 h.
1. Secondly, the prepared quartz sand was added into above solution. The system was stirred and heated up to 70 °C using a water bath for 15 min followed by dried for 2.5 h at 110 °C. Thirdly, the reaction product was immersed in deionized water for 24 h and washed several times with deionized water to eliminate the influence of physical adsorption. Lastly, the surface modified filter was obtained by dried at 110 °C for 12 h.
2.5 Preparation of GOS
KHS was mixed with GO–Cl in pyridine, then stirring at 0 °C for 12 h. The coated sand was washed with distilled water until the runoff was clear, dried at 60 °C, and stored in capped bottles.2.6 Column test on NS and GOS
A mini-pilot-scale equipment was constructed and used in this study (Fig. 1c). For the column tests, the adsorption column (5.2 mm diameter × 310 mm long) was filled with NS (Fig. 1a) or GOS (Fig. 1b), and the feed solution was flowed through the column at controlled flow rate, the eluted solution was continuous collected every 1 hour and the flow rate is 1 mL min−1.2.7 Characterization
Atomic force microscope (AFM) observation of GO sheets was recorded using a Vecco Digital Instrument Multimode V scanning probe microscope, in which samples prepared by spin-coating sample solutions onto freshly exfoliated silicon substrates. Fourier-transform infrared (FT-IR) spectra were recorded on a ProStar LC240 (Varian, USA). Scanning electron microscopy (SEM) images were taken on a Hitachi S-47000 field-emission SEM system. Thermal gravimetric analysis (TGA) was carried out on a Perkin-Elmer Pyris 6 TGA instrument at a heating rate of 20 °C min−1 under nitrogen flow. Raman spectra were collected on a Jobin-Yvon Raman spectroscope equipped with a 514 nm laser source (HR800, France). The turbidity was measured with a turbidimeter Hanna model HI93703. Dissolved organic carbon (DOC) was measured with TOC analyzer (TOC-VCPH, Shimadzu, Japan). UV254 was measured with spectrometer (UV754, CANY, China). The concentrations of Cd2+ and Pb2+ were determined by ICP-MS.3. Results and discussion
3.1 Synthesis and characterization of GO and GOS
The concentration of carboxylic acid groups in the GO was estimated by acid–base titration. Before titration, the GO were stirred in water for 2 days under argon and collected via filtration to remove acid residue. In a typical titration experiment, pretreated GO (80 mg) were stirred in 50.00 mL of 0.05 M aqueous NaOH solution under argon for 48 h. The mixture was filtered through a 1.2 μm pore-size membrane and washed with deionized water until the filtrate was neutral. The combined filtrate and washings were titrated with 34.50 mL of 0.05 M aqueous HCl solution to reach the neutral point (pH = 7.00), as monitored by a pH meter, and thus the content of acidic sites in the GO is calculated to be 0.78 mmol. The carbon content of the GO is estimated to be 6.67 mmol by assuming that the GO is solely composed of carbon, and thus the molar ratio of the acidic sites in the GO sample is 11.6%.In our experiments, GO–Cl was firstly synthesized from GO (Fig. 2), because the acyl chloride on the surface of GO are highly reactive. It is easy to react with modified sand, so GO can binds strongly to the surface of quartz sand (Fig. 4). The molecular formula of KH550 modifier and its chemical changes during hydrolysis are shown in Fig. 3a. During hydrolysis of the silane, the SiOC2H5 group will transform into Si–OH. We hypothesize that the surface modification mechanism is carried on the mode given in Fig. 3b.29
AFM is a direct method to characterize the morphologies and the thickness of GO. Fig. 5 shows the tapping mode AFM images of GO. The thickness of GO is about 0.8 nm (Fig. 5b), which agrees with other researchers' reports,30,31 suggesting the complete exfoliation of graphite oxide into individual GO sheet.
The FTIR spectra of NS, KHS and GOS are shown in Fig. 6. From Fig. 6a we can see the asymmetry stretching vibration and deformation vibration of Si–O–Si at 1200 cm−1 to 1100 cm−1 and 472 cm−1, which indicated the formation of quartz sand filter. From Fig. 6b we can see that the absorption peaks at 2935 cm−1, 2893 cm−1 and 3320 cm−1 ascribed to asymmetry stretching vibration, symmetry stretching vibration of C–H and stretching vibration of N–H, respectively, which demonstrates that KH550 exists in quartz sand surface. In the spectra of Fig. 6c, the characteristic absorptions of amide II (at 1550 cm−1) were clearly observed, it can be concluded that the GO has been incorporated into the surface of sand. Moreover, the physical adsorbed KH550 and GO were washed with deionized water for several times in surface modification process, so the physical adsorption between quartz sand and GO can be eliminated.
The Raman spectra of GOS sample in Fig. 7 show the characteristic peaks located at 1339 and 1598 cm−1 corresponding to the D and G bands of graphene sheets, respectively,32 while barely any features are obtained from NS spectrum.
In this work, XPS analysis of the quartz sand before and after surface modified were employed to confirm the interface bonding way between graphene and the quartz sand surface. The results of the XPS analyses are shown in Fig. 8. Table 1 shows the detailed data corresponding to Fig. 8. It is shown in Fig. 8 that nitrogen and carbon content are not present at the surface of the unmodified quartz sand. In contrast, KHS contains nitrogen and carbon. Furthermore, there is a significant depletion in oxygen content and a slight decrease in silicon content on the KH550 modified quartz sand surface in comparison to the unmodified quartz sand, while for GOS, the carbon content increased to 52.43. This is further affirmed that the GO is well bind to the surface of quartz sand.
| Element | NS | KHS | GOS |
|---|---|---|---|
| Si (%) | 29.25 | 20.56 | 12.36 |
| O (%) | 70.75 | 47.76 | 35.21 |
| C (%) | — | 31.68 | 52.43 |
| N (%) | — | 0.22 | 0.14 |
Fig. 9 corresponds to SEM images of NS, KHS and GOS. Specifically, Fig. 9a shows the extremely rough and uneven of the unmodified quartz sand surface, while the KH550 modified quartz sand surface possesses comparatively a flatter surface (Fig. 9b) due to a thin layer of KH550 grafted on the surface of quartz sand filter. In the Fig. 6c and d, we can see that a lot of graphene coating on the surface of quartz sand and more tiny pores appear on its surface. This result is accordant with the analyses results of XPS and FT-IR.
TGA curves of the NS, KHS and GOS are shown in Fig. 10. The TGA data for GOS showing a weight loss of ∼8.0% contrasted with that of NS. Comparatively, 7% weight loss of KHS could be observed, which can strongly prove that GO has grafted on the sand surface.
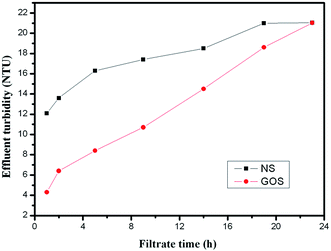
3.2 Effectiveness of the GOS for pollutants removal
| Time (h) | Turbidity (NTU) | DOC (mg L−1) | UV254 (cm−1) |
|---|---|---|---|
| 1 | 8.4 | 2.55 | 0.132 |
| 2 | 9.2 | 3.24 | 0.148 |
| 5 | 10.5 | 4.01 | 0.152 |
| 7 | 11.2 | 4.25 | 0.167 |
| 9 | 13.8 | 4.31 | 0.171 |
| 12 | 14.9 | 4.73 | 0.182 |
| 15 | 15.7 | 4.81 | 0.193 |
| 18 | 17.5 | 4.85 | 0.198 |
| 21 | 19.6 | 4.96 | 0.216 |
| 23 | 20.6 | 5.02 | 0.221 |
4. Conclusions
In this work, we synthesized graphene–sand composites using acyl chloride-functionalized GO and modified quartz sand, GO firmly combined in the surface of quartz sand by chemical bond. This creates a new kind of porous carbon-based multi-functional adsorption material. Through a series of adsorption test, we conclude that the nanostructured GO coating can significantly increase the retention of turbidity, organic matter and heavy metals over the parent sand granules. This kind of technique combine together the excellent function of nanostructures material with the traditional quartz sand, and have very widely applied foreground in the water treatment.Acknowledgements
This research was supported by the plan of basic applied study of Suzhou (SYG201417) and the Fundamental Research Funds for the Central Universities (JB2013115).Notes and references
- M. Machida, T. Mochimaru and H. Tatsumoto, Carbon, 2006, 44, 2681 CrossRef CAS PubMed.
- C. L. Chen and X. K. Wang, Ind. Eng. Chem. Res., 2006, 45, 9144 CrossRef CAS.
- C. Moreno-Castilla and M. A. Alvarez-Merino, Langmuir, 2004, 20, 8142 CrossRef CAS PubMed.
- J. Goel, K. Kadirvelu, C. Rajagopal and V. K. Garg, Ind. Eng. Chem. Res., 2006, 45, 6531 CrossRef CAS.
- A. L. Bojic, D. Bojic and T. Andjelkovic, J. Hazard. Mater., 2009, 168, 813 CrossRef CAS PubMed.
- N. Fatemi and B. Sarkar, Environ. Health Perspect., 2002, 110, 695 CrossRef CAS.
- B. D. Shoener, I. M. Bradley, R. D. Cusick and J. S. Guest, Environ. Sci.: Processes Impacts, 2014, 16, 1204 CAS.
- X. Zhou, Y. X. Li and Y. Zhao, RSC Adv., 2014, 4, 15620 RSC.
- S. Karthikeyan, C. Anandan, J. Subramanian and G. Sekaran, RSC Adv., 2013, 3, 15044 RSC.
- Z. Alouini and M. Jemli, J. Environ. Monit., 2001, 3, 548 RSC.
- H. Cao, W. T. Qu and X. L. Yang, Anal. Methods, 2014, 6, 3799 RSC.
- A. Sengupta, P. K. Mohapatra, M. Iqbal, W. Verboom, J. Huskens and S. V. Godbole, RSC Adv., 2012, 2, 7492 RSC.
- J. R. Maxwell, C. T. Pillinger and G. Eglinton, Q. Rev., Chem. Soc., 1971, 25, 571 RSC.
- W. Z. Bao, F. Miao, Z. Chen, H. Zhang, W. Y. Jang, C. Dames and C. N. Lau, Nat. Nanotechnol., 2009, 4, 562 CrossRef CAS PubMed.
- C. Wang, Y. Liu, L. Li and H. Tan, Nanoscale, 2014, 6, 5703 RSC.
- Y. B. Zhang, Y. W. Tan, H. L. Stormer and P. Kim, Nature, 2005, 438, 201 CrossRef CAS PubMed.
- C. Lee, X. D. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385 CrossRef CAS PubMed.
- W. Q. Fan, Q. H. Zhang and Y. Wang, Phys. Chem. Chem. Phys., 2013, 15, 2632 RSC.
- X. Deng, L. LÜ, H. Li and F. Luo, J. Hazard. Mater., 2010, 183, 923 CrossRef CAS PubMed.
- G. Zhao, L. Jiang, Y. He, J. Li, H. Dong, X. Wang and W. Hu, Adv. Mater., 2011, 23, 3959 CrossRef CAS PubMed.
- A. L. Masters, B. J. Vinci, B. Brazil, D. A. Creaser and S. T. Summerfelt, Aquacultural Engineering, 2008, 38, 66 CrossRef PubMed.
- L. C. Ram and R. E. Masto, Earth-Sci. Rev., 2014, 128, 52 CrossRef CAS PubMed.
- I. B. Bame, J. C. Hughes, L. W. Titshall and C. A. Buckley, Chemosphere, 2013, 93, 2171 CrossRef CAS PubMed.
- G. X. Zhao, J. X. Li, X. M. Ren, C. L. Chen and X. K. Wang, Environ. Sci. Technol., 2011, 45, 10454 CrossRef CAS PubMed.
- W. Gao, M. Majumder, L. B. Alemany, T. N. Narayanan, M. A. Ibarra, B. K. Pradhan and P. M. Ajayan, ACS Appl. Mater. Interfaces, 2011, 3, 1821 CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- N. I. Kovtyukhova, P. J. Ollivier, B. R. Martin, T. E. Mallouk, S. A. Chizhik, E. V. Buzaneva and A. D. Gorchinskiy, Chem. Mater., 1999, 11, 771 CrossRef CAS.
- W. J. Hou, B. Q. Tang, L. L. Lu, J. Sun, J. J. Wang, C. X. Qin and L. X. Dai, RSC Adv., 2014, 4, 4848 RSC.
- H. Y. Li, R. G. Wang, H. L. Hu and W. B. Liu, Appl. Surf. Sci., 2008, 255, 1894 CrossRef CAS PubMed.
- X. Q. Zhang, X. Y. Fan, H. Z. Li and C. Yan, J. Mater. Chem., 2012, 22, 24081 RSC.
- Z. Xu and C. Gao, Macromolecules, 2010, 43, 6716 CrossRef CAS.
- R. Saito, K. Sato, P. T. Araujo, D. L. Mafra and M. S. Dresselhaus, Solid State Commun., 2013, 175, 18 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |