A supramolecular probe for colorimetric detection of Pb2+ based on recognition of G-quadruplex†
Abstract
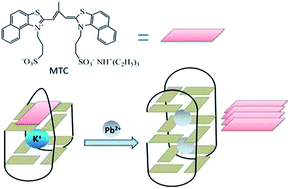
A colorimetric probe of Pb2+ has been designed based on the mechanism that a supramolecular probe selectively recognized the Pb2+-induced conformational transition of G-quadruplexes. The probe exhibited high sensitivity and selectivity towards Pb2+, which enabled it to be practically used in detection of the Pb2+ level in a freshwater system.

 Please wait while we load your content...
Please wait while we load your content...