Molecular dynamics simulation of the cooperative effect by different force fields in monosodium glutamate aqueous solution
Abstract
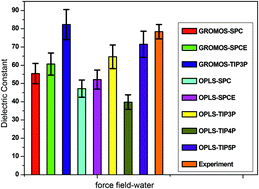
Different force fields (GROMOS and OPLS) in conjunction with different water models (SPC, SPCE, TIP3P, TIP4P and TIP5P) were assessed using molecular dynamics simulations of monosodium glutamate (MSG) aqueous solution. The dielectric constant obtained using GROMOS with TIP3P matches with the experimental results from dielectric spectroscopy (DRS) with minimum error. The structural information of MSG aqueous solution was also investigated. It was found that the hydration shells of water around charged particles (Na+ and Glu−) highly influence the dielectric constant of the solution. In addition to the first hydration shell, the second shell also affects the dielectric constant. Glu− weakened the hydrogen bond (HB) lifetime between water molecules in MSG aqueous solution compared to the water–water HB lifetime in pure water. It seems that a 10 ns simulation is not long enough to accurately determine the dielectric constant using the GROMOS force field with the TIP3P model of water.

 Please wait while we load your content...
Please wait while we load your content...