pH responding reversible supramolecular self-assembly of water-soluble amino-imidazole-armed perylene diimide dye for biological applications†
Abstract
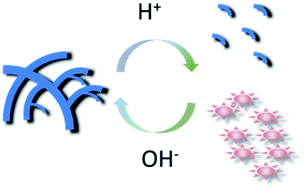
It is extremely important to design stimuli-responsive biomimetic supramolecular materials. Such type of materials require molecular monomers with multiple functionalities. Perylene diimide (PDI) has been considered as one of the most versatile building block units for supramolecular architecture. However, most of the reported PDI derivatives work in organic media, whereas their application in aqueous systems is a challenge due to the pronounced hydrophobicity of their perylene backbones. Here, we report a water-soluble amino-imidazole-armed perylene diimide (AIA-PDI) dye that discloses reversible supramolecular structure and fluorescence emission conversion upon external pH-stimulation. Such characteristics offer a gap of PDI derivatives in the fabrication of a pH-responsive biomimetic system. Successful application for glucose detection, as a proof of concept, further demonstrates this PDI derivative's biological suitability in pH-responsive systems.

 Please wait while we load your content...
Please wait while we load your content...