Polymer impregnated sulfonated carbon composite solid acid catalyst for alkylation of phenol with methyl-tert-butyl ether†
Praveen K. Khatri,
Manvi Manchanda,
Indrajit K. Ghosh and
Suman L. Jain*
Chemical Sciences Division, CSIR-Indian Institute of Petroleum, Dehradun-248005, India. E-mail: suman@iip.res.in; Fax: +91-135-2660202; Tel: +91-135-2525788
First published on 2nd December 2014
Abstract
A polymer impregnated sulfonated carbon composite solid acid (P–C–SO3H) catalyst was synthesized via sulfonation of a composite material formed through incomplete carbonization of hydrolyzed glucose supported on a polymer matrix (co-polymer of styrene and chloromethylstyrene i.e. Merrifield's peptide resin) and used for the alkylation of phenol using methyl-tert-butyl ether (MTBE) as an alkylating agent in a pressure reactor under autogenous pressure. The developed catalyst exhibited excellent catalytic activity and provided para-tert-butyl phenol (PTBP) exclusively with the added benefits of facile recovery and reusability for several runs without loss of catalytic activity.
Introduction
p-tert-Butyl phenol (PTBP) is a stable and readily biodegradable chemical which is being widely used as an intermediate for phenol resins and polycarbonate resins.1,2 It is also used as a raw material for construction elements and floors in buildings. Additionally t-butylated phenols (TBPs) have extensively been used as precursors or starting materials in a variety of industries including pharmaceuticals. For instance, the product o-tert-butylphenol (OTBP) is an intermediate for pesticides, fragrances and other products, whereas PTBP is used as a raw material for the production of a variety of resins, phosphate esters, durable surface coatings, varnishes, wire enamels, fragrances, oil field chemicals and demulsifiers. 2,4-Di-tert-butylphenol (DTBP) is an intermediate for antioxidants and 2,6-di-tert-butylphenol is used as an antioxidant intermediate and in pharmaceuticals.3–12 Conventional processes for producing tert-butyl phenols (TBPs) from an isobutylene-containing C4 raffinate stream typically involve introducing methanol, or another alcohol, into the C4 stream to react and produce alkyl tertiary butyl ether intermediate that is further decomposed to make high-purity isobutylene, which can then be reacted with phenol to produce the tert-butyl phenol. The additional step of adding methanol significantly increases the costs of producing tert-butyl phenols and creates another undesirable byproduct. Besides the additional expenses, the additional byproducts are unattractive for environmental reasons. Isobutylene may also be produced by isobutanol dehydration. However, in this case an impure product may form which, when used for alkylating phenols, leads to the formation of phenol by-products, such as derivatives having a secondary butyl substitute.13,14 Thus, it is advantageous to use MTBE as an alkylating agent which generates pure isobutylene in situ and the co-product methanol can be easily recovered and recycled.Owing to the growing environmental considerations, the use of solid acid catalysts is preferred as they can easily be recovered, recycled and can eliminate the problems associated with the highly corrosive and polluting homogeneous liquid acid catalysts.15–19 Heterogeneous acid catalysts studied so far for the tert-butylation of phenol using MTBE as an alkylating agent include cation-exchange resins,20 zeolites,7,21–23 clay-based catalysts24 and mesoporous molecular sieves.25–27 Cation-exchange resins owing to their easy availability and low cost have been considered to be most promising, however, the physical strength of the ion exchange resins is lowered at a high temperature and therefore, the reaction must be carried out at lower temperature (100–120 °C) that lead to the poor yield of the desired 4-TBPs. Recently sulfonated carbons (C–SO3H) derived from the incomplete carbonization of simple sugars such as glucose has come out to be a new class of catalysts exhibiting excellent catalytic performance for acid catalyzed reactions such as biodiesel synthesis. Recently, Goodwin et al.28 reported a novel polymer impregnated sulfonated carbon based solid acid catalysts for esterification reactions. Inspired with this report, we thought it would be worthwhile to explore the potential of such catalysts for other industrially important acid catalyzed reactions. Accordingly, herein we report an efficient polymer impregnated sulfonated carbon composite solid acid catalyst for the alkylation of phenol using MTBE as an alkylating agent in a pressure reactor under autogenous pressure. The reaction was found to be highly regioselective and afforded PTBP selectively in excellent yield.
Results and discussion
Synthesis and characterization of the catalyst
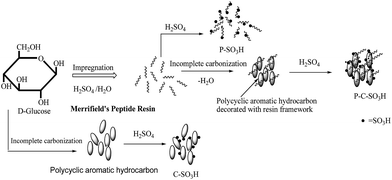
During the present study, the targeted polymer impregnated sulfonated carbon (P–C–SO3H) was synthesized via sulfonation of a composite material formed through incomplete carbonization of hydrolyzed glucose supported in a polymer matrix based on a copolymer of styrene and chloromethylstyrene i.e. Merrifield resin (Scheme 1). For the comparative study, the corresponding sulfonated carbon catalyst (C–SO3H) was prepared via incomplete carbonization of glucose and a polymer-based catalyst without carbon (P–SO3H) was prepared by the sulfonation of the incompletely carbonized Merrifield resin.The powder X-ray diffraction of the synthesized P–C–SO3H catalyst did not show any characteristic peak and exhibited a broad peak centered at 2θ value of 25°, indicating that the synthesized material is amorphous in nature (Fig. S1†).
FT-IR spectra of the synthesized P–C–SO3H and C–SO3H catalysts are shown in Fig. S2.† A broad absorption band around 3300 cm−1 in P–C–SO3H obtained after sulfonation was attributed to OH stretching with two shoulder peaks due to the aromatic C–H and aliphatic C–H stretching. The band around 1630–1780 cm−1 corresponding to the overlapped C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and S
O and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching was stronger in P–C–SO3H catalyst than in C–SO3H, most likely due to the higher loading of the acid sites in the polymer impregnated carbon catalyst. The poor acidity of carbon (C–SO3H) catalyst was further proved by a very weak band around 1200 cm−1 corresponding to the asymmetric and symmetric stretching of sulfonic acid groups. This peak was found to be quite stronger in P–C–SO3H catalyst. Furthermore, a sharp absorption band near to 600 cm−1 corresponding to C–Cl and C–S stretching was appeared in P–C–SO3H.
O stretching was stronger in P–C–SO3H catalyst than in C–SO3H, most likely due to the higher loading of the acid sites in the polymer impregnated carbon catalyst. The poor acidity of carbon (C–SO3H) catalyst was further proved by a very weak band around 1200 cm−1 corresponding to the asymmetric and symmetric stretching of sulfonic acid groups. This peak was found to be quite stronger in P–C–SO3H catalyst. Furthermore, a sharp absorption band near to 600 cm−1 corresponding to C–Cl and C–S stretching was appeared in P–C–SO3H.
Sulfur contents or SO3H-acid densities in P–C–SO3H, C–SO3H and P–SO3H catalysts were estimated by elemental analysis. The acidity of the P–C–SO3H, C–SO3H and P–SO3H calculated from elemental sulfur analysis by assuming that all the sulfur atoms in the catalyst are presented in –SO3H form were found to be 2.44, 1.28 and 1.52 mmol H+ per g respectively. In addition, the total acidity of the synthesized catalysts was determined using standard acid–base back titration method.29 The acid site densities as calculated by titration method were found to be slightly higher (2.54 mmol H+ per g for P–C–SO3H and 1.36 mmol H+ per g for C–SO3H) in comparison to the estimated values obtained from elemental sulfur analysis data. This discrepancy in results may be due to the generation of phenolic-OH and –COOH groups by incomplete carbonization of glucose.30,31 The higher acidity of P–C–SO3H catalyst is most likely due to the presence of evenly distributed polycyclic aromatic hydrocarbons along to the resin framework which may lead to the greater availability of active sites for sulfonation and resulted to the higher acid density of P–C–SO3H as compared to the C–SO3H and P–SO3H.28 The BET surface areas along with the SO3H-acid site densities of the synthesized catalysts are given in the Table 1.
| Catalyst | BET surface area (m2 g−1) | S-contenta (mmol g−1) | Total acid densityb (mmol H+ per g) |
|---|---|---|---|
| a Determined by elemental analysis.b Determined by acid–base titration method (dried catalyst (0.1 g) was treated with aq. NaOH (0.004 N, 30 ml) for 0.5 h at room temperature under stirring. After that a pH titrator was used to neutralize excess NaOH with 0.02 N HCl). | |||
| P–C–SO3H | <1 | 2.44 | 2.54 |
| C–SO3H | <1 | 1.28 | 1.36 |
| P–SO3H | <1 | 1.52 | 1.53 |
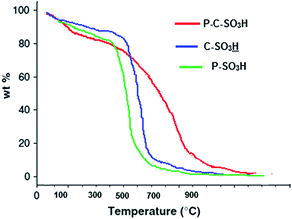
Thermal stability of the synthesized sulfonated catalysts was determined by thermal gravimetric analysis (TGA). As shown in Fig. 1, the initial weight loss (about 5%) below 150 °C in all three catalysts was mainly due to the loss of a small amount of adsorbed water. The weight loss process of P–C–SO3H and C–SO3H below 150 °C was found to be similar, however at higher temperatures (>300 °C) P–C–SO3H showed a plateau indicating a much slower rate of weight loss as compared to that of C–SO3H, which is mainly due to the gradual desorption and thermal decomposition process of polymer framework (Fig. 1). However, the thermogravimetric curve of P–SO3H showed a significant weight loss in the range of 350–550 °C due to the degradation of polymer framework (Fig. 1). Finally a rapid weight loss was occurred in the temperature range 550 to 650 °C when no mass was left at this temperature.
Catalytic activity
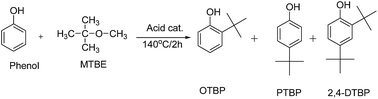
The catalytic activity of the synthesized P–C–SO3H, C–SO3H and P–SO3H catalysts was checked for the alkylation of phenol with MTBE in a high pressure reactor under autogenous pressure to give tert-butyl phenol derivatives. A general reaction scheme for the alkylation of phenol is shown in Scheme 2.Isobutylene thus generated from the acid catalyzed cracking of MTBE reacts with phenol and gave tert-butyl phenols such as o-tert-butyl phenol (OTBP), p-tert-butyl phenol (PTBP) and 2,4-di-tert butyl phenol (2,4-DTBP) without any evidence for the formation of 2,6-di-tert-butyl phenol and any other side product which may produce by oligomerization of isobutylene. The results of these experiments are given in Table 2. As shown, among all three catalysts studied, polymer impregnated sulfonated carbon (P–C–SO3H) catalyst was found to be superior and gave excellent conversion of phenol with the formation of p-tert-butyl phenol as the predominant product (Table 2, entry 4). In case of C–SO3H, the reaction rate was found to be very slow and gave only 10% conversion with the 62% selectivity for the PTBP (Table 2, entry 13). The sulfonated polymer based catalyst (P–SO3H) showed comparable initial activity as P–C–SO3H for the alkylation of phenol but lost about 20% of its activity in the subsequent run (Table 2, entry 14). The higher reactivity of P–C–SO3H compared to that of other two sulfonated catalysts can be explained on the basis of its higher SO3H acid site densities. Based on these findings, we have chosen P–C–SO3H catalyst for the further studies.
| Entry | Molar ratio (PhOH + MTBE) | T/(°C) | Cat. (wt%) | Conv.b (%) | Selectivity of productc (%) | ||
|---|---|---|---|---|---|---|---|
| OTBP | PTBP | 2,4-DTBP | |||||
| a Catalyst P–C–SO3H; reaction time 2 h.b Determined by GC and GCMS.c Determined by GC and GCMS.d Using C–SO3H as catalyst.e Using P–SO3H catalyst.f Recycling of the P–SO3H catalyst.g Using similar reactant/catalytic acid site ratio (P–C–SO3H).h Using similar reactant/catalytic acid site ratio (C–SO3H).i Using similar reactant/catalytic acid site ratio (P–SO3H).j Using the reaction mixture obtained at 110 °C as the reactant. | |||||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
110 | 5 | 93 | 15 | 36 | 44 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
120 | 5 | 93 | 17 | 42 | 36 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 5 | 95 | 17 | 48 | 31 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
140 | 5 | 96 | 5 | 80 | 15 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 5 | 96 | 5 | 81 | 13 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
160 | 5 | 96 | 6 | 80 | 12 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
140 | 1 | 82 | 13 | 61 | 18 |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
140 | 3 | 89 | 5.5 | 68 | 26.5 |
| 9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
140 | 7 | 95 | 7 | 80 | 13 |
| 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
140 | 5 | 96 | 5 | 79 | 16 |
| 11 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
140 | 5 | 95 | 6 | 80 | 14 |
| 12 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
140 | 5 | 94 | 8 | 66 | 20 |
| 13d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
140 | 5 | 10 | 12 | 62 | 26 |
| 14e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
140 | 5 | 80, 58f | 14, 18 | 66, 62 | 20 |
| 15g,h,i | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
140 | 1.46 mmol SO3H | 96g, 30h, 89i | 5, 15, 18 | 80, 72, 68 | 15, 13, 14 |
| 16j | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 5 | 95 | 6 | 79 | 15 |
| 17j | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
160 | 5 | 96 | 5 | 81 | 14 |
In order to find out the best reaction conditions with P–C–SO3H catalyst a number of controlled experiments were performed under different reaction conditions. The results of these experiments are summarized in Table 2 (entries 1–12). The reaction temperature was found to be a key parameter for alkylation of phenol with respect to the selectivity of the desired p-tert-butyl phenol (PTBP) as shown in Table 2, entries 1–6). When the reaction was performed at 110 °C under otherwise identical conditions, the selectivity of PTBP was very low. The selectivity of PTBP was appreciably improved with increasing the temperature from 120 to 140 °C (entries, 2–4). Further increase of temperature from 150 °C to 160 °C (entry 5 and 6) did not show any noticeable boost in the selectivity of PTBP. Based on these findings 140 °C was considered to be optimum reaction temperature for this reaction at which maximum yield of PTBP was obtained. Similarly the amount of catalyst had pronounced effect on the conversion of phenol (Table 1, entries 4, 7–9). It was noticed that the conversion of phenol was significantly increased with increasing the catalyst quantity from 1 to 5 wt% (entries 4, 7–8). The maximum conversion of phenol was achieved (i.e. 96%) at 5 wt% of the catalyst and beyond this no significant change in the conversion of phenol was obtained (entry 9). All the experiments were carried out by using the molar ratio of phenol and MTBE as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. An increase in molar ratio of phenol to MTBE from 2 to 5 did not show any significant improvement in the conversion of phenol and selectivity of PTBP or in product distribution ratio (entries 10–11). Similarly, no further improvement was observed when 2
1. An increase in molar ratio of phenol to MTBE from 2 to 5 did not show any significant improvement in the conversion of phenol and selectivity of PTBP or in product distribution ratio (entries 10–11). Similarly, no further improvement was observed when 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of phenol and MTBE was used (Table 2, entry 12). Apart from the comparative studies based on same wt% of all three catalysts, we also performed the comparison using same phenol/MTBE/catalytic site molar ratio. Considering that all the sulfur is presented in the form of –SO3H, we have used 1.46 mmol –SO3H, which is equivalent to the (0.6 g of P–C–SO3H, 1.14 g of C–SO3H and 0.96 g of P–SO3H when 1
1 molar ratio of phenol and MTBE was used (Table 2, entry 12). Apart from the comparative studies based on same wt% of all three catalysts, we also performed the comparison using same phenol/MTBE/catalytic site molar ratio. Considering that all the sulfur is presented in the form of –SO3H, we have used 1.46 mmol –SO3H, which is equivalent to the (0.6 g of P–C–SO3H, 1.14 g of C–SO3H and 0.96 g of P–SO3H when 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of phenol and MTBE was used). The results of these experiments are summarized in Table 2 (entry 15). As shown, the higher activity of all the catalysts might be due to the presence of nearly equal active catalytic acid sites. However, the superiority of the P–C–SO3H catalyst can be explained on the basis of higher loading and stability of –SO3H groups without showing any leaching during the reaction. Moreover we also performed the experiments using the reaction mixture obtained at 110 °C as the reactant for the alkylation at higher temperatures i.e. 150 and 160 °C under otherwise identical experimental conditions (Table 2, entries 16–17). The product distribution ratio was remained almost similar to that of using fresh reactants.
1 molar ratio of phenol and MTBE was used). The results of these experiments are summarized in Table 2 (entry 15). As shown, the higher activity of all the catalysts might be due to the presence of nearly equal active catalytic acid sites. However, the superiority of the P–C–SO3H catalyst can be explained on the basis of higher loading and stability of –SO3H groups without showing any leaching during the reaction. Moreover we also performed the experiments using the reaction mixture obtained at 110 °C as the reactant for the alkylation at higher temperatures i.e. 150 and 160 °C under otherwise identical experimental conditions (Table 2, entries 16–17). The product distribution ratio was remained almost similar to that of using fresh reactants.
Multiple recycling experiments were performed to examine the reusability and the stability of the –SO3H groups in the polymer composite catalyst (P–C–SO3H). At the end of the reaction, the catalyst was recovered by simple filtration and reused for the subsequent alkylation of phenol with MTBE under described reaction conditions. The recycling of the recovered catalyst was tested for six runs (Fig. 2). In these experiments the conversion of phenol and selectivity for PTBP remained as good as with the fresh catalyst, indicating that the synthesized catalyst is highly stable and can be reused for several runs. Furthermore, the sulfur content of the recovered catalyst obtained after six runs was found to be almost similar to the fresh one. These results suggested the higher stability of the SO3H groups in P–C–SO3H and confirmed that the developed catalyst is truly heterogeneous in nature. To compare the stability of –SO3H groups, we also checked the recycling ability of C–SO3H catalyst under similar reaction conditions. The catalyst exhibited almost negligible activity for the second run and gave <2% conversion of phenol. Furthermore, significantly lower sulfur content in the recovered catalyst indicating the less stability of –SO3H groups due to the desulfonation in C–SO3H catalyst.
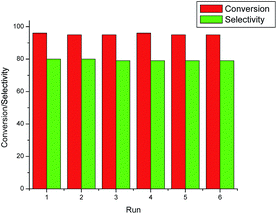 | ||
| Fig. 2 Recycling experiments red bar indicates the conversion of phenol and green bar is attributed to the selectivity of PTBP. | ||
Experimental
Reagents used
Reagents including D-glucose (reagent grade, Aldrich), Merrifield's peptide resin, 1% cross linked, 100–200 mesh (Sigma-Aldrich), acetonitrile (CH3CN, 99.9%, Acros Organics), conc. sulfuric acid (99.7%, Aldrich) were used as received.Preparation of the polymer impregnated-SO3H functionalized carbon composite
Polymer impregnated –SO3H containing carbon composite (P–C–SO3H) was synthesized by the pyrolysis of a polymer matrix impregnated with D-glucose and followed by its sulfonation with conc. sulfuric acid as depicted in Scheme 1. In a typical experiment, a solution of D-glucose (2.0 g) in 3 ml of deionized water was added with 2 drops of sulfuric acid (H2SO4) and the resulting mixture was slowly added to a pre dried Merrifield's peptide resin (2 g) with magnetic stirring. After complete addition of glucose solution, the resulting mixture was dried at 110–120 °C for 12 h. Thus obtained black material was crushed to powder and then pyrolyzed under dry nitrogen at 300 °C for 1 h in a muffle furnace.The resulting black residue was subjected to sulfonation by using concentrated sulfuric acid (1 g solid/20 ml H2SO4) at 160 °C for 12 h under nitrogen atmosphere. The mixture was diluted with plenty of distilled water and the black material so obtained was collected by filtration, washed several times with hot distilled water of 80 °C until impurities like sulfate ions were no longer detected in the wash water. The resulting black solid (P–C–SO3H) was dried in an oven at 80 °C under vacuum for 5 h and subsequently used for the alkylation of phenol with MTBE. For the comparative studies, a sulfonated polymer based solid acid (P–SO3H) catalyst was prepared via direct sulfonation of Merrifield peptide resin and sulfonated carbon catalyst (C–SO3H) was synthesized by the heating of D-glucose powder (10 g) at 400 °C for 1 h under dry N2 atmosphere by incomplete carbonization. The resulting black material was crushed to powder and then subjected to sulfonation with conc. H2SO4. Sulfonated polymer matrix (P–SO3H) was prepared by heating of Merrifield's peptide resin at 300 °C for 1 h under dry N2, followed by its sulfonation at 160 °C for 13 h under a dry N2 atmosphere.
General procedure for the alkylation of phenol
The alkylation experiments were carried out in a stainless steel batch reactor of 25cc and 100cc by varying the reaction parameters under autogenous pressure conditions. In a typical experiment phenol (6.2 g, 66 mmol) and MTBE (5.8 g, 66 mmol) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio and catalyst P–C–SO3H (5 wt% of the total weight of reactants) were charged into a 100cc parr autoclave. The mixture was heated at 140 °C for 2 h under stirring. At the end of the reaction, the vessel was cooled down to room temperature and the catalyst was separated by filtration. The recovered catalyst was washed with methanol, dried and used for the recycling experiments. Filtrate so obtained was diluted with acetonitrile and subjected for gas chromatography (GC) and GCMS analysis to determine the conversion and the selectivity of the TBPs as shown in Table 2.
1 molar ratio and catalyst P–C–SO3H (5 wt% of the total weight of reactants) were charged into a 100cc parr autoclave. The mixture was heated at 140 °C for 2 h under stirring. At the end of the reaction, the vessel was cooled down to room temperature and the catalyst was separated by filtration. The recovered catalyst was washed with methanol, dried and used for the recycling experiments. Filtrate so obtained was diluted with acetonitrile and subjected for gas chromatography (GC) and GCMS analysis to determine the conversion and the selectivity of the TBPs as shown in Table 2.
Conclusion
In summary, we have demonstrated a highly efficient solid acid catalyst, easily prepared via sulfonation of a polymer impregnated carbon composite for alkylation of phenol using MTBE as the alkylating agent under mild experimental conditions. The reaction was found to be highly selective and afforded p-tert-butyl phenol predominantly in excellent yield. The developed polymer impregnated carbon based solid acid was found to be superior in terms of both activity and stability in comparison to the sulfonated carbon (C–SO3H) and sulfonated polymer (P–SO3H) catalyst. The prepared catalyst could easily be recovered and recycled for several runs without any significant loss in activity. No leaching of active –SO3H groups was observed as evidenced from elemental sulfur analysis of recovered catalyst makes the present method superior and more advantageous than existing ones.Acknowledgements
We sincerely thank to Director, IIP for his kind permission to publish these results. We are also thankful to analytical sciences division of the Institute for providing help and support in analyses of the samples.Notes and references
- S. E. Dapurkar and P. Selvam, Appl. Catal., A, 2003, 254, 239–249 CrossRef CAS.
- J. H. Huang, L. H. Xing, H. S. Wang, G. Li, S. J. Wu, T. H. Wu and Q. B. Kan, J. Mol. Catal. A: Chem., 2006, 259, 84–90 CrossRef CAS PubMed.
- T. Mathew, B. S. Rao and C. S. Gopinath, J. Catal., 2004, 222, 107–116 CrossRef CAS PubMed.
- K. Ojha, N. C. Pradhan and A. C. Samanta, Chem. Eng. J., 2005, 112, 109–115 CrossRef CAS PubMed.
- J. Gui, H. Ban, X. Cong, X. Zhang, Z. Hu and Z. Sun, J. Mol. Catal. A: Chem., 2008, 225, 27–31 CrossRef PubMed.
- A. Sakthivel, S. K. Badamali and P. Selvam, Microporous Mesoporous Mater., 2000, 39, 457 CrossRef CAS.
- A. V. Krishnan, K. Ojha and N. C. Pradhan, Org. Process Res. Dev., 2002, 6, 132 CrossRef CAS.
- K. Zhang, H. Zhang, G. Xua, S. Xiang, D. Xu, S. Liu and H. Li, Appl. Catal., A, 2001, 207, 183 CrossRef CAS.
- T. Sato, G. Sekiguchi, T. Adschiri and K. Arai, Chem. Commun., 2001, 1566 RSC.
- S. Sumbramanian, A. Mitra, C. V. V. Satyanarayana and D. K. Chakrabarty, Appl. Catal., A, 1997, 159, 229 CrossRef.
- S. H. Patinuin and B. S. Friedman, in Alkylation of aromatics with alkenes and alkanes in Friedel Crafts and related reactions, ed. G. A. Olah, Interscience, New York, 1964, vol. 3, p. 75 Search PubMed.
- I. F. Lorenc, G. Lambeth and W. Scheffer, in Kirk-Othmer Encyclopedia of Chemical Technology, ed. M. Howe-Grant and J. I. Kroschwitz, Wiley, New York, 1992, vol. 2, p. 113 Search PubMed.
- G. D. Yadav and N. Kirthivasan, in Fundamental and Applied Aspects of Chemically Modified Surfaces, ed. J. P. Blitz and C. B. Little, Royal Society of Chemistry, UK, 1999, pp. 254–269 Search PubMed.
- G. D. Yadav, A. A. Pujari and A. V. Joshi, Green Chem., 1999, 1, 269 RSC.
- A. A. Carlton, J. Org. Chem., 1948, 13, 120 CrossRef.
- P. Elavarasan, K. Kondamudi and S. Upadhyavula, Chem. Eng. J., 2011, 166, 340–347 CrossRef CAS PubMed.
- Y. G. Li, B. Xue and Y. T. Yang, Fuel Process. Technol., 2009, 90, 1220–1225 CrossRef CAS PubMed.
- L. Ronchin, A. Vavasori and L. Toniolo, J. Mol. Catal. A: Chem., 2012, 355, 134–141 CrossRef CAS PubMed.
- G. D. Yadav and G. S. Pathre, Microporous Mesoporous Mater., 2006, 89, 16–24 CrossRef CAS PubMed.
- K. G. Chandra and M. M. Sharma, Catal. Lett., 1993, 19, 309–317 CrossRef CAS.
- E. Dumitriu and V. Hulea, J. Catal., 2003, 218, 249–257 CrossRef CAS.
- R. Anand, R. Maheswari, K. U. Gore and B. B. Tope, J. Mol. Catal. A: Chem., 2003, 193, 251–257 CrossRef CAS.
- Z. K. Zhao, W. L. Wang, W. H. Qiao, G. R. Wang, Z. S. Li and L. B. Cheng, Microporous Mesoporous Mater., 2006, 93, 164–170 CrossRef CAS PubMed.
- G. D. Yadav and N. S. Doshi, Appl. Catal., A, 2002, 236, 129–147 CrossRef CAS.
- S. K. Badamali, A. Sakthivel and P. Selvam, Catal. Lett., 2000, 65, 153–157 CrossRef CAS.
- C. H. Subrahmanyam, B. Viswanathan and T. K. Varadarajan, J. Mol. Catal. A: Chem., 2005, 226, 155–163 CrossRef CAS PubMed.
- T. Jiang, Y. Ma, J. Cheng, W. Liu, X. Zhou, Q. Zhao and H. Yin, J. Assoc. Arab Univ. Basic Appl. Sci., 2014 DOI:10.1016/j.jaubas.2014.01.003.
- X. Mo, E. Lotero, C. Lu, Y. Liu and J. G. Goodwin, Catal. Lett., 2008, 123, 1–6 CrossRef CAS.
- S. P. J. Higson, Analytical Chemistry, Oxford Univ. Press, New York, 2003, p. 75 Search PubMed.
- A. Takagaki, M. Toda, M. Okamura, J. N. Kondo, S. Hayashi, K. Domen and M. Hara, Catal. Today, 2006, 116, 157 CrossRef CAS PubMed.
- M. Okamura, A. Takagaki, M. Toda, J. N. Kondo, T. Tatsumi, K. Domen, M. Hara and S. Hayashi, Chem. Mater., 2006, 18, 3039 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra11033a |
| This journal is © The Royal Society of Chemistry 2015 |