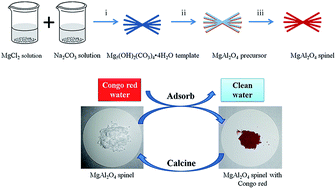
A porous MgAl2O4 spinel (MAS) has been synthesized via a facile hard template process with further calcination, in which a hierarchical Mg5(OH)2(CO3)4·4H2O template with a high surface area was used as a hard template and magnesium source, and AlCl3·6H2O was used as an alumina source. The as-prepared samples were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), energy dispersive X-ray (EDX), thermogravimetry analysis (TGA) and N2 adsorption–desorption. The MAS samples showed superior adsorption performance, including rapid adsorption rate, excellent adsorption capacity and good reusability for removal of Congo red (CR) from aqueous solution. The superior performance could be ascribed to the strong interaction between the MAS samples and CR, as well as the hierarchical porous structure and the high specific surface area of the samples. The maximum adsorption capacity of the MAS samples for CR was nearly 845 mg g−1, which is higher than most of the previously reported adsorbents. The CR removal process was found to follow the pseudo-second-order rate equation and the Sips adsorption model.