Fabrication of nanostructured V2O5 via urea combustion for high-performance Li-ion battery cathode†
Qiang Songa,
Hongchang Panga,
Weitao Gonga,
Guiling Ning*a,
Song Gaob,
Xinglong Dongb,
Chunjing Liub,
Junying Tiana and
Yuan Lina
aState Key Laboratory of Fine Chemicals and Faculty of Chemical, Environmental & Biological Science and Technology, Dalian University of Technology, 2 Linggong Road, High Technology Zone, Dalian 116012, P. R. China. E-mail: ninggl@dlut.edu.cn; Fax: +86-411-84986067; Tel: +86-411-84986067
bSchool of Materials Science and Engineering, Dalian University of Technology, Dalian, 116012, P. R. China
First published on 18th November 2014
Abstract
Nanostructured vanadium pentoxide (V2O5) crusts were facilely synthesized via the combustion of a precursor by mixing commercial V2O5 with molten urea. The nanocrusts were transferred to nanorods during further annealing at 630 °C. Both the V2O5 nanocrusts and V2O5 nanorods were used preliminarily as a cathode material for Li-ion batteries. Their electrode performance was highly improved compared to commercial V2O5.
Vanadium pentoxide (V2O5) has attracted considerable attention due to its great potential in a variety of applications, such as electrode materials in solid state batteries and supercapacitors.1–6 In particular, V2O5 is one of the most promising cathode materials for high energy density Li-ion batteries owing to its high theoretical capacity (440 mA h g−1 with three lithium insertions/extractions), which is much higher than those of more widely used cathode materials, such as LiCoO2 (140 mA h g−1), LiMnO2 (148 mA h g−1), and LiFePO4 (170 mA h g−1).5–8 V2O5 also has the additional advantages of being inexpensive and abundant. On the other hand, the slow diffusion of lithium ions (D ∼ 10−12 cm2 s−1) and low electronic conductivity (10−2 to 10−3 S cm−1) of commercial V2O5 crystals limit their use as high-performance electrode materials for Li-ion batteries in large scale production.7–10 Recently, many studies indicate that nanostructures of electrode materials can improve the performance due to the dimension reduction of particles size, which reduces the distance of ions diffusion and electron transportation.9–11
Up to now, various V2O5 materials with different nanostructures, such as nanorods, nanofibers, nanowires, and nanobelts have been prepared by chemical methods, such as hydrothermal growth, sol–gel synthesis and flame spray-pyrolyzed.8–15 For instance, nanorod structured V2O5 synthesized by the thermal decomposition of vanadium precursors, exhibiting a good discharge capacity and cycle stability as a cathode for Li batteries.9 In addition, nanorods, nanowires and nanobelts have also been obtained by thermal evaporation, sputtering deposition and recrystallization, even though the products of these methods mainly are thin layers on substrates.16,17 Glushenkov et al. fabricated V2O5 nanorods via long time ball milling and annealing, giving a stable electrochemical performance as a cathode for Li batteries.16 The main focus has been on the development of a convenient method that can be applied to produce V2O5 nanomaterials in large scale production. Therefore, there is a great need for a novel cost-effective method producing V2O5 nanomaterials with high performance for Li-ion battery.
In this study, we report a novel and convenient approach for producing nanostructured V2O5 crusts (nanocrusts), which is feasible for mass production. The V2O5 nanocrusts composed of nanoparticles were fabricated via the direct combustion of a black precursor at 450 °C in air for an hour. A black precursor was obtained by commercial V2O5 reacting with molten urea at a starting mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 at 140 °C for 30 min. Herein, urea is not only the combustion agent but also the reagent for the reaction of V2O5 in preprocessing.18,19 V2O5 nanorods were obtained by a recrystallization process during further annealing at 630 °C. The electrochemical performance of the as-prepared V2O5 electrode materials for Li-ion batteries was demonstrated, exhibiting high-rate charge–discharge capacity and good cycle stability.
4 at 140 °C for 30 min. Herein, urea is not only the combustion agent but also the reagent for the reaction of V2O5 in preprocessing.18,19 V2O5 nanorods were obtained by a recrystallization process during further annealing at 630 °C. The electrochemical performance of the as-prepared V2O5 electrode materials for Li-ion batteries was demonstrated, exhibiting high-rate charge–discharge capacity and good cycle stability.
An image of the black precursor via the reaction of commercial V2O5 and molten urea is shown in Fig. S1a.† The colour of the melt mass changed gradually from yellow (commercial V2O5) to black, indicating that the V2O5 had been reacted with molten urea. The FT-IR spectrum of the black precursor is shown in Fig. S2.† The bands at about 3461, 3340, 2226, 1682, 1605, 1466, 1338, 1156, 981, and 574 cm−1 were indexed to the black precursor. The bands at 1682 and 1466 cm−1 were ascribed to stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–N, while those bands at 3461, 3340 and 1605 cm−1 can be assigned to asymmetric, symmetric stretching and deformation vibration of N–H bonds, respectively. The band at 1156 cm−1 can be attributed to the rocking vibration of NH2. These can be attributed to the presence of unreacted urea in the black precursor. The absorption at 981 cm−1 should be associated with the V
O and C–N, while those bands at 3461, 3340 and 1605 cm−1 can be assigned to asymmetric, symmetric stretching and deformation vibration of N–H bonds, respectively. The band at 1156 cm−1 can be attributed to the rocking vibration of NH2. These can be attributed to the presence of unreacted urea in the black precursor. The absorption at 981 cm−1 should be associated with the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band, which is related to vanadic complex after the reaction of V2O5 and molten urea.20,21 All the three major absorption peaks of V2O5 at 617, 827, and 1022 cm−1 were invisible in the spectrum (Fig. S1a†).22 Moreover, the bands at 2226 and 1338 cm−1 can be ascribed to the absorptions of –N
O band, which is related to vanadic complex after the reaction of V2O5 and molten urea.20,21 All the three major absorption peaks of V2O5 at 617, 827, and 1022 cm−1 were invisible in the spectrum (Fig. S1a†).22 Moreover, the bands at 2226 and 1338 cm−1 can be ascribed to the absorptions of –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. These indicate that V2O5 has completely reacted with urea.20–22
O. These indicate that V2O5 has completely reacted with urea.20–22
The thermal decomposition behaviour of the precursor was investigated by thermogravimetric (TG) analysis. Fig. S3† shows the TG results for the black precursor calcined in air. Two distinct stages were observed. The sharp weight loss around 200 °C in the first stage can be attributed to the condensation and deamination of urea in the mixed precursor.18 A steep slope indicating another weight loss at 405 °C was then observed, which demonstrated the successful thermal-decomposition of vanadate to vanadium oxide. The weight of the final product corresponds to the V2O5 in the initial mass ratio.19
The micro morphologies and structures of commercial V2O5 and the V2O5 nanocrusts are shown in Fig. 1a and b. As shown in Fig. 1a, the commercial V2O5 is micrometer-sized particles with irregular shapes. The yellow bread-like product with a foam structure (see Fig. S1b and c†) is obtained by calcinating the black precursor in air at 450 °C for 1 h. Fig. S1c† shows that the foam structure consists of nanostructured crusts (nanocrusts) during combustion. Fig. 1b indicates that the V2O5 nanocrusts are composed of nanoparticles. Furthermore, elongated nanorods have been formed by further annealing of the nanocrusts in air at 630 °C (Fig. 1d). The nanocrusts can also be crushed into individual nano-sized particles by grinding. The morphology and structure of the nanoparticles obtained by simply grinding the nanocrusts are shown in Fig. 1c and e; the size of the particles ranged from 50 nm to 300 nm. Fig. 1f shows a high resolution TEM image of a part of the nano-sized V2O5 particles, revealing a layer structure of V2O5. The lattice fringes with a spacing of 0.58 nm should correspond to the (200) planes of orthorhombic V2O5.23
The phase and purity of the as-prepared nanocrusts and nanorods were determined by powder X-ray diffraction (XRD). The diffraction patterns are displayed in Fig. 2. Both of them can be indexed well to a monocline structure V2O5 space group with the space group: P21/c, a = 11.512 Å, b = 3.564 Å, c = 4.368 Å (JCPDS card no. 65-0131), showing the high phase purity of the nanocrusts and the nanorods. The peaks of the V2O5 nanocrusts were weaker and wider than those of the V2O5 nanorods, particularly the relative intensity of (001) peak, which suggests that the nanorods have better crystallinity and a larger particle size. The crystallite dimensions of nanocrusts and nanorods can be calculated from the (001) peak via the Scherrer equation. The V2O5 nanocrusts (36.7 nm) is much smaller than the V2O5 nanorods (78.5 nm).9,16
The morphologies of the products obtained from different urea contents in the starting reagents are shown in Fig. 3. Fig. 3a and b show SEM images of the samples produced by thermal decomposition at 450 °C for 1 h with a mass ratio of V2O5 to urea of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1
5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, respectively. The morphologies of the samples are nanocrusts assembled from nanoparticles. However, the nanostructured crusts cannot be obtained in the 1
4, respectively. The morphologies of the samples are nanocrusts assembled from nanoparticles. However, the nanostructured crusts cannot be obtained in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 V2O5 products (mass ratio of V2O5 to urea is 1
3 V2O5 products (mass ratio of V2O5 to urea is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Instead, micro-aggregates were observed (Fig. 3c). The V2O5 bread could be formed eventually with excess urea decomposition during the calcination process. The nanocrusts are the bubble walls in V2O5 bread. Therefore, the thinner crust would be achieved in a higher urea mass ratio, because the formation of more gas from urea decomposition leads to larger bubbles and thinner walls in V2O5 bread.
3). Instead, micro-aggregates were observed (Fig. 3c). The V2O5 bread could be formed eventually with excess urea decomposition during the calcination process. The nanocrusts are the bubble walls in V2O5 bread. Therefore, the thinner crust would be achieved in a higher urea mass ratio, because the formation of more gas from urea decomposition leads to larger bubbles and thinner walls in V2O5 bread.
 | ||
Fig. 3 SEM images of the V2O5 nanocrusts synthesized by thermal decomposition of the black precursor with different starting molar ratios of V2O5 to urea: (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; (b) 1 5; (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; (c) 1 4; (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. 3. | ||
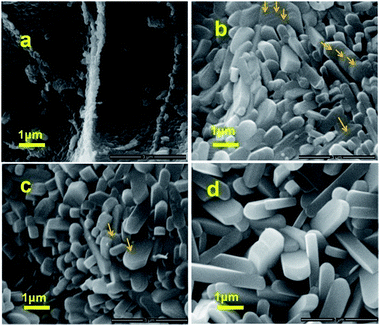
The shape transformations of the V2O5 nanocrusts by further annealing progressing at 630 °C are shown in Fig. 4. The SEM image in Fig. 4a shows that the surface of the nanocrusts consists of nanoparticles with irregular shapes. The SEM images in Fig. 4b–d reveal the morphological changes in the nanocrusts after 5, 10 and 30 min of the annealing treatment. The shapes of the powders are transformed almost completely from nanocrusts to nanorods after 30 min annealing. These nanorods look like ice cream sticks, which have a round end and rectangular cross section. The width of the rods grown after 5 min was in the range of 300–800 nm. The thickness was between 100 and 300 nm. The length was up to several micrometers. According to Glushenkov et al.,16 the formation and growth of nanorods can be attributed to the surface energy driven recrystallization process. The morphology of the nanorods is a particular type of facet that is dominated by notable low energy {001} surfaces (by calculation).24 As shown in Fig. 4b and c, new layers (shown with arrows) were formed on the {001} surfaces in the nanorod growth process.16 In addition, the nanocrusts provide enough space for particle growth to elongated nanorods, preventing the formation of larger crystals due to the coalescence on adjacent nanorods.
This method involving a reaction between commercial V2O5 and molten urea and annealing in a muffle furnace is easy to produce real mass quantities of materials with a nanostructure. The products can be broken to nanocrusts, nanoparticles or nanorods by ball milling. If required, materials with various particle sizes can be separated by sieving. Both the roasting technology and mechanical milling can be easily capable of scaling up.
Considering the Li-ion storage performance of commercial V2O5, we investigated the applications in a Li ion battery (LIB) as a proof-of-concept demonstration of their potential use.
Galvanostatic charge–discharge measurements were performed on the V2O5 nanocrusts and the V2O5 nanorods at a current density of 30 mA g−1 between 2.0 and 4.0 V. The initial five charge–discharge profiles for these measurements are illustrated in Fig. 5a and b. Both of the nanocrusts and nanorods showed three characteristic plateaus at the voltages of about 3.4, 3.2 and 2.3 V (vs. Li/Li+) in those discharge curves. These plateaus correspond to the phase transformation of crystalline α-V2O5 to α-LixV2O5 (x < 0.01), ε-LixV2O5 (0.35 < x < 0.7), δ-LixV2O5 (0.7 < x < 1), and γ-LixV2O5 (1 < x < 1.9), respectively.2,25 Cyclic voltammetry (CV) of the nanocrusts (Fig. S6a†) between 1.5 and 4 V was measured at a scan rate of 1 mV s−1. The pairs of redox peaks can be resolved clearly in the voltage window of 1.5–4.0 V, which is consistent with the plateaus of the discharge voltage/capacity profile. In previous studies, the electrochemical reaction between vanadium oxides and metal lithium can be described as follows:2,25–28
| V2O5 + 0.5Li+ + 0.5e− ⇌ Li0.5V2O5 | (1) |
| Li0.5V2O5 + 0.5Li+ + 0.5e− ⇌ LiV2O5 | (2) |
| LiV2O5 + Li+ + e− ⇌ Li2V2O5 | (3) |
The first discharge capacity of the V2O5 nanocrusts is more than 294 mA h−1, which is more than the theoretical capacity for 2Li+ intercalation. The large capacity can be attributed to the nanocrusts, which have a large interfacial area with the electrolyte and defects in the V2O5 materials formed during thermal decomposition of the precursor. These can lead to more than two Li+ insertions in the voltage window of 2.0–4.0 V. As shown in Fig. S7,† the discharge capacity of the V2O5 nanocrusts and the V2O5 nanorods maintain a stable capacity around 220 and 180 mA h g−1, respectively. Compared to that of the commercial V2O5 (the discharge capacity is ∼70 mA h g−1), the discharge capacity of the V2O5 nanocrusts and the V2O5 nanorods are much higher.
Fig. 5c shows the specific capacity of the first 50 cycles at a current density of 300 mA g−1 between 2.0 and 4.0 V (versus Li+/Li) for commercial V2O5, the V2O5 nanocrusts and the V2O5 nanorods, respectively. The V2O5 nanocrusts exhibit an initial capacity of 353 mA h g−1 and 210 mA h g−1 during the 1st and 30th cycle. The discharge capacity of the V2O5 nanorods only deliver about 160 mA h g−1 after 30 cycles, which can be attributed to the growth of V2O5 crystals increasing the Li+ diffusion path length.29 Although there is some loss of the capacity for the V2O5 nanocrusts and V2O5 nanorods, the capacity of V2O5 nanocrusts and V2O5 nanorods is 2–3 times of the capacity of commercial V2O5 after 50 cycles. The capacity loss could be attributed to the phase of a crystalline V2O5 irreversible transition during insertion/extraction of Li+ ions. These cyclic performances are better than the nano-sized V2O5 synthesized by a thermal treatment or flame spray pyrolysis.13,30 The results of the V2O5 nanocrusts are also better than that of vanadium oxide nanobelts prepared by a hydrothermal method.12,20,31,32 Compared to other transition metal oxides, such as MnO2 and Co3O4, the discharge capacity of the as-obtained nanostructured V2O5 is still good as a cathode material owing to its high theoretical capacity.1,33
The rate capability of the electrode materials for LIB is also an important factor for practical applications. To investigate the rate capability of the V2O5 nanocrusts and the V2O5 nanorods, galvanostatic cycling was carried out at various current densities. Fig. 5d shows the rate performance of the V2O5 nanocrusts and the V2O5 nanorods over the voltage range of 2.0–4.0 V (versus Li+/Li) at different current densities in the range of 30–3000 mA g−1. The discharges of both samples decreased with increasing current density. Similar to the cycle performance, the nanocrusts V2O5 showed a relatively enhanced rate capability compared to the nanorods V2O5. An initial discharge capacity of 410 mA h g−1 is attained at a low current density of 30 mA g−1 for the nanocrusts V2O5, which stabilizes at above 300 mA h g−1 after 6 cycles. The discharge capacities of the nanocrusts V2O5 are about 257, 237, 185, and 85 mA h g−1, at 300, 600, 1000, and 3000 mA g−1, respectively. Correspondingly, the nanorods V2O5 have values of 330, 178, 124, 89, and 21 mA h g−1 at different rates of 30, 300, 600, 1000, and 3000 mA g−1, respectively. Owing to the inherent low electronic conductivity of V2O5, the discharge capacities of both samples are greatly reduced at higher current densities.2 Furthermore, the discharge capacities of both samples recovered to 250 and 205 mA h g−1, upon returning to a current of 30 mA g−1. This shows that both of the structural stability and reversibility for the two samples are quite good. The rate performance of the V2O5 nanocrusts is comparable to the previously reported carbon-coated vanadium oxide.30,34 These results also indicate that the electrochemical property of the V2O5 nanocrusts and the V2O5 nanorods are close to the results of nano-structured vanadium oxide reported previously.13,23–29 On the other hand, this method is more facile and capable of scaling up for broad application prospects.
Conclusions
A potential method for the fabrication of the V2O5 nanocrusts has been proposed via the combustion of precursor from mixing commercial V2O5 with molten urea. The V2O5 nanorods were further prepared through a controllable nanoscale growth originating from the nanocrusts during further annealing at 630 °C. The minimization of surface energy of V2O5 grain probably drives the growth of the nanorods dominated by the {001} surface. The discharge capacity of the as-obtained the V2O5 nanocrusts and the V2O5 nanorods are increased to 210 and 170 mA h g−1, respectively, from 70 mA h g−1 after 30 cycles compared to commercial V2O5.Acknowledgements
This work was supported by the National High-tech R & D Program (863 Program, no. 2008AA03Z306), the National Natural Science Foundation of China (51003009, 21276046 and 21476045), the Ministry of Education Science and technology research projects, the Fundamental Research Funds for the Central Universities of China (DUT11LK16), (DUT13RC(3)043 and DUT14RC(3)040). We also thank Professor Zhang Xinbo (Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun) for electrochemical measurements.Notes and references
- J. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- Y. Wang and G. Cao, Chem. Mater., 2006, 18, 2787–2804 CrossRef CAS.
- H. Liu and W. Yang, Energy Environ. Sci., 2011, 4, 4000 CAS.
- S. Batteries, S. Tepavcevic, H. Xiong, V. R. Stamenkovic, X. Zuo and M. Balasubramanian, ACS Nano, 2012, 6, 530–538 CrossRef CAS PubMed; F. Wang, S. Xiao, Y. Hou, C. Hu, L. Liu and Y. Wu, RSC Adv., 2013, 3, 13059 RSC.
- H. Pang, Y. Dong, S. L. Ting, J. Lu, C. M. Li, D.-H. Kim and P. Chen, Nanoscale, 2013, 5, 7790–7794 RSC.
- S. Wang, Z. Lu, D. Wang, C. Li, C. Chen and Y. Yin, J. Mater. Chem., 2011, 21, 6365 RSC.
- M. Armand and J. Tarascon, Nature, 2008, 451, 2–7 CrossRef PubMed.
- T. Zhai, H. Liu, H. Li, X. Fang, M. Liao, L. Li, H. Zhou, Y. Koide, Y. Bando and D. Golberg, Adv. Mater., 2010, 22, 2547–2552 CrossRef CAS PubMed; X. Rui, J. Zhu, W. Liu, H. Tan, D. Sim, C. Xu, H. Zhang, J. Ma, H. H. Hng, T. M. Lim and Q. Yan, RSC Adv., 2011, 1, 117 RSC.
- A. Pan, J.-G. Zhang, Z. Nie, G. Cao, B. W. Arey, G. Li, S. Liang and J. Liu, J. Mater. Chem., 2010, 20, 9193 RSC.
- Y. Wang and G. Cao, Adv. Mater., 2008, 20, 2251–2269 CrossRef CAS.
- X. Yu, Z. Lu, G. Zhang, X. Lei, J. Liu, L. Wang and X. Sun, RSC Adv., 2013, 3, 19937 RSC.
- W. Hu, X.-B. Zhang, Y.-L. Cheng, Y.-M. Wu and L.-M. Wang, Chem. Commun., 2011, 47, 5250–5252 RSC.
- N. Pinna, J.-F. Hochepied, M. Niederberger and M. Gregg, Phys. Chem. Chem. Phys., 2009, 11, 3607 RSC.
- H. Pang, P. Cheng, H. Yang, J. Lu, C. X. Guo, G. Ning and C. M. Li, Phys. Chem. Chem. Phys., 2013, 49, 1536–1538 CAS.
- H. Li, P. He, Y. Wang, E. Hosono and H. Zhou, J. Mater. Chem., 2011, 21, 10999 RSC.
- A. M. Glushenkov, V. I. Stukachev, M. F. Hassan, G. G. Kuvshinov, H. K. Liu and Y. Chen, Cryst. Growth Des., 2008, 2–6 Search PubMed.
- C. K. Chan, H. Peng, R. D. Twesten, K. Jarausch, X. F. Zhang and Y. Cui, Nano Lett., 2007, 7, 490–495 CrossRef CAS PubMed.
- Y.-C. Si, L.-F. Jiao, H.-T. Yuan, H.-X. Li and Y.-M. Wang, J. Alloys Compd., 2009, 486, 400–405 CrossRef CAS PubMed.
- Y. L. Cheah, V. Aravindan and S. Madhavi, J. Electrochem. Soc., 2012, 159, A273–A280 CrossRef CAS PubMed.
- B. Azambre, M. J. Hudson and O. Heintz, J. Mater. Chem., 2003, 13, 385–393 RSC.
- H. Wang, K. Huang, C. Huang, S. Liu, Y. Ren and X. Huang, J. Power Sources, 2011, 196, 5645–5650 CrossRef CAS PubMed.
- P. Liu, I. L. Moudrakovski, J. Liu and A. Sayari, Chem. Mater., 1997, 4756, 2513–2520 CrossRef CAS; S. Cheng, H.-D. Hwang and G. E. Maciel, J. Mol. Struct., 1998, 470, 135–149 CrossRef.
- X. Zhou, G. Wu, J. Wu, H. Yang, J. Wang, G. Gao, R. Cai and Q. Yan, J. Mater. Chem. A, 2013, 1, 15459 CAS.
- D. C. Sayle, D. H. Gay, A. L. Rohl, C. R. A. Catlow, J. H. Harding and M. A. Perrid, J. Mater. Chem., 1996, 6, 653–660 RSC.
- C. O'Dwyer, V. Lavayen, D. a. Tanner, S. B. Newcomb, E. Benavente, G. González and C. M. S. Torres, Adv. Funct. Mater., 2009, 19, 1736–1745 CrossRef.
- D. Yu, C. Chen, S. Xie, Y. Liu, K. Park, X. Zhou, Q. Zhang, J. Li and G. Cao, Energy Environ. Sci., 2011, 4, 858 CAS.
- Q. An, Q. Wei, L. Mai, J. Fei, X. Xu, Y. Zhao, M. Yan, P. Zhang and S. Huang, Phys. Chem. Chem. Phys., 2013, 15, 16828–16833 RSC.
- D. Fang, L. Li, W. Xu, G. Li, Z. Luo, C. Liang, Y. Ji, J. Xu and C. Xiong, RSC Adv., 2014, 4, 25205 RSC.
- X. Chen, E. Pomerantseva, K. Gregorczyk, R. Ghodssi and G. Rubloff, RSC Adv., 2013, 3, 4294 RSC.
- J. Shin, H. Jung, Y. Kim and J. Kim, J. Alloys Compd., 2014, 589, 322–329 CrossRef CAS PubMed.
- X. Zhou, G. Wu, J. Wu, H. Yang, J. Wang and G. Gao, Phys. Chem. Chem. Phys., 2014, 16, 3973–3982 RSC.
- L. Mai, L. Xu, C. Han, X. Xu, Y. Luo, S. Zhao and Y. Zhao, Nano Lett., 2010, 10, 4750–4755 CrossRef CAS PubMed.
- B. Li, H. Cao, J. Shao, G. Li, M. Qu and G. Yin, Inorg. Chem., 2011, 50, 1628–1632 CrossRef CAS PubMed.
- N. S. Ergang, J. C. Lytle, K. T. Lee, S. M. Oh, W. H. Smyrl and a. Stein, Adv. Mater., 2006, 18, 1750–1753 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supplemental data of Fourier transform infrared spectrum, thermal gravimetric analysis, electron microscopy images and cycle discharge performance. See DOI: 10.1039/c4ra11015c |
| This journal is © The Royal Society of Chemistry 2015 |