Ultrasensitive and stable determination of lead ions by a glassy carbon electrode modified with a phenanthroline-based electropolymerized film†
C. Y. Jia,
P. Lia,
H. W. Maa,
G. C. Yangb and
M. Zhang*a
aState Key Laboratory of Supramolecular Structure and Materials, Jilin University, Changchun 130012, P. R. China
bSchool of Chemistry and Life Science, Changchun University of Technology, Changchun 130012, P. R. China. E-mail: zhming@jlu.edu.cn; Fax: +86-431-85193421; Tel: +86-431-85167507
First published on 12th November 2014
Abstract
A novel electro-active compound, 3,8-bis(9,9-bis(6-(9H-carbazol-9-yl)hexyl)-9H-fluoren-2-yl)-1,10-phenanthroline (TCFC), is used to modify a glassy carbon electrode (GCE) for the electrochemical detection of Pb2+. The phenanthroline unit in the backbone and four electro-active alkyl-linked peripheral carbazole groups as the side chains endow TCFC with metal-chelating ability and the possibility of electropolymerization. By the method of chemical preconcentration/absorption square wave anodic stripping voltammetry (SWASV), the TCFC electropolymerized film-modified GCE (TCFC/GCE) shows excellent selectivity and sensitivity towards Pb2+, even in the presence of a large amount of other heavy metal ions (HMIs). The calculated limit of detection (LOD) towards Pb2+ could be improved to 1.33 × 10−11 M (S/N = 3) under optimum conditions. Moreover, TCFC/GCE is stable under ambient conditions (>30 days) and can be recycled while retaining high sensitivity, which is of great practical value. Further, TCFC/GCE can be applied to the determination of Pb2+ in real water samples.
1. Introduction
Lead ions (Pb2+) are nondegradable and can accumulate in bones, muscles, the kidneys and the brain, resulting in brain damage, mental retardation, behavioral problems and so on.1–3 Therefore, the sensitive and robust detection of trace Pb2+ in medicine, the environment and biology is exigent.The electrochemical detection of Pb2+ has some attractive features, such as high selectivity and sensitivity, intrinsically simple operation, robustness and inexpensiveness.4,5 Additionally, chemically modified electrodes (CMEs) have their own particular advantage, in that they can preconcentrate trace heavy metal ions (HMIs) during the accumulation step.6 Thus, CMEs have been widely used for determination of trace HMIs. Though considerable efforts have been made to develop CMEs for HMIs sensing,7–10 a stable and sensitive CME for Pb2+ detection is still highly needed, and this possibility deserves to be explored.
The electropolymerization (EP) method causes the electro-active precursors to undergo the oxidative coupling reaction, resulting in direct polymerization on the glassy carbon electrode (GCE). EP has the advantages of controlling morphology and conductivity by judicious selection of the precursors and/or potentiometric conditions. The resulting cross-linking network of the EP film is beneficial for application in the sensing field, due to the stability that is possible and the rapid diffusion of analytes. Thus, electrodes modified by EP materials have excellent potential to detect HMIs.
In this paper, we report a novel electro-active material, TCFC, which is used as the CME modifier to detect Pb2+. The chemical structure of TCFC is shown in Scheme 1. As can be seen, 1,10-phenanthroline (PHEN) in the backbone could endow TCFC with strong metal-chelating capability, acting as a receptor for the molecular recognition of Pb2+. Meanwhile, the alkyl-linked peripheral carbazole groups in the side chains – carbazole being a well-known electro-active precursor – could provide the crossing site under anodic oxidation (the 3- and 6- active sites could form dimers), resulting in the cross-linking network film. Based on this molecular design, TCFC can be coated onto a GCE (TCFC/GCE) by the EP method. TCFC/GCE exhibits high sensitivity towards Pb2+, and the calculated limit of detection (LOD) towards Pb2+ can be down to 1.33 × 10−11 M; such a low LOD is the best level achieved in Pb2+ sensing publications, which can be attributed to the strong chelating ability of PHEN in TCFC and the intrinsic microstructure of EP films.11 Furthermore, TCFC/GCE is stable under ambient conditions and can be recycled while retaining high sensitivity (continuous measurement for 30 days). The TCFC/GCE sensor is also capable of determining Pb2+ in real samples without any significant interference or matrix problems.
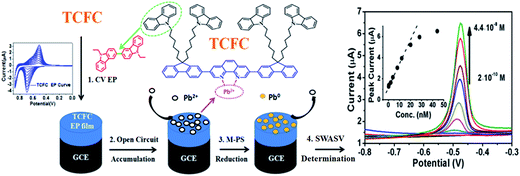 | ||
| Scheme 1 The chemical structure of TCFC and illustration of the EP and Pb2+ SWASV detection process using TCFC/GCE. | ||
2. Experimental section
2.1 Chemicals and apparatus
All the reagents and solvents used for the synthesis were purchased from Sigma Aldrich or Acros and used as received.Electrochemical measurements were carried out with a CHI 660C electrochemical workstation. Electrochemical impedance spectroscopy (EIS) images were taken by a Princeton Applied Research impedance meter (PARSTAT 2273). Fluorescence spectra were recorded by a fluorescence spectro-photometer (Shimadzu RF-5301 PC) equipped with a xenon lamp excitation source. 1H NMR spectra were recorded on a Bruker AVANCZ 500 spectrometer at 500 MHz using DMSO as solvent at 298 K and tetramethylsilane (TMS) as the internal standard. All aqueous solutions were prepared using Milli-Q water of 18.2 MΩ (Millipore) resistivity. The pH values were measured by a pH meter (METTLER TOLEDO LE438). The 0.1 M acetate buffer solution (pH 4.5) was prepared by adding 0.1 M sodium acetate to 0.1 M acetic acid. Pb2+ solutions were prepared by diluting the appropriate amount of Pb2+ stock solution (0.1 M). Indium tin oxide (ITO) used for AFM measurements was cleaned in an ultrasonic bath with toluene, acetone, ethanol, and deionized water, and dried with nitrogen.
2.2 Synthesis of TCFC
The synthetic route for TCFC has been reported.12 17.5 mg Pd(PPh3)4 was added to a mixture of 3,8-dibromo-1,10-phenanthroline (0.03 g, mmol) and 2-(4,4,1,5,5-tetramethyl-1,3,2-dioxaborolan)-9,9-(N-carbazole-hexyl)fluorene (150 mg, mmol) in 3 mL of toluene and 2 mL of 2.0 M Na2CO3 under a nitrogen atmosphere, and the resulting mixture was stirred at 80 °C for 72 h. After cooling to room temperature, the mixture was extracted with dichloromethane. The organic layer was dried over anhydrous MgSO4, The precipitated solid was filtrated and purified by chromatography using petroleum ether/dichloromethane as the eluent to afford a white solid (0.06 g, yield: 50%). 1H NMR (500 MHz, DMSO): δ 9.55 (d, 2H), 8.84 (d, 2H), 8.11 (d, 2H), 8.06 (d, 8H), 8.02 (s, 2H), 7.97 (m, 4H), 7.86 (m, 2H), 7.45 (d, 8H), 7.34 (m, 12H), 7.27 (m, 2H), 7.1 (t, 8H), 4.23 (t, 8H), 2.1 (m, 8H), 1.52 (m, 8H), 1.03 (m, 16H), 0.50 (m, 8H). MALDI-TOF-MS (m/z): 1506.01 [M+] 1507.3.2.3 Preparation of TCFC/GCE (step 1 of Scheme 1)
Firstly, the GCE was polished with different grades of alumina powder (0.05, 0.3 and 1.0 μm) on nylon cloth, emery paper and chamois, respectively, rinsed with deionized water and then cleaned in an ultrasonic bath for 2 min, in accordance with literature procedures (unless otherwise stated).13Then, the cyclic voltammetry (CV) method was used to prepare an EP film on the GCE by using a standard one-compartment, three-electrode electrochemical cell. Titanium metal and GCE were used as the counter electrode and the working electrode, respectively. The reference electrode was a Ag/Ag+ nonaqueous electrode. A mixture of TCFC precursor molecule (0.5 mg mL−1) and TBAPF6 with CH2Cl2 and CH3CN (v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was used as the electrolyte solution. The TCFC electro-active precursor was electropolymerized through an oxidative coupling reaction (0 to 0.85 V) to form the cross-linking network film on the GCE (TCFC/GCE).
3) was used as the electrolyte solution. The TCFC electro-active precursor was electropolymerized through an oxidative coupling reaction (0 to 0.85 V) to form the cross-linking network film on the GCE (TCFC/GCE).
Lastly, TCFC/GCE was washed with a mixture of CH2Cl2 and CH3CN (v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to remove the surface electrolyte and precursor, and then the prepared TCFC/GCE was dried in a vacuum oven at 40 °C for three hours.
3) to remove the surface electrolyte and precursor, and then the prepared TCFC/GCE was dried in a vacuum oven at 40 °C for three hours.
2.4 Electrochemical detection experiments (steps 2, 3 and 4 of Scheme 1)
Electrochemical detection experiments were carried out in a standard one-compartment, three-electrode electrochemical cell. TCFC/GCE, Pt wire electrode and Ag/AgCl (3 M KCl) aqueous electrode were used as the working electrode, the counter electrode and the reference electrode, respectively. Preconcentration of Pb2+ (step 2 of Scheme 1) took place under open circle conditions by dipping the working electrode into HAc/NaAc aqueous buffer (0.1 M, pH 4.5) containing Pb2+ with vigorous stirring for 300 s, followed by rinsing with deionized water three times. Then, these electrodes were immersed in a 10 mL electrochemical cell containing 5 mL HAc/NaAc buffer solution (0.1 M, pH 4.5). Multi potential steps (M-PS) (step 3 of Scheme 1) were performed at a potential of −1.2 V for 30 s. After that, a SWASV scan (step 4 of Scheme 1) was performed from −1.0 V to 0.4 V by using the following waveform parameters: the scan frequency was 25 Hz, the amplitude was 25 mV and the step increment was 5.0 mV (unless stated otherwise).In addition, with the aim of the recycled use of TCFC/GCE, TCFC/GCE was immersed into aqueous 2,2′,2′′,2′′′-(ethane-1,2-diyldinitrilo)tetraacetic acid (EDTA) and scanned at 0.4 V for 1 min under vigorous stirring to clean off any remaining traces of Pb2+ for the next use.
2.5 Real world water sample analysis
The real world water samples were taken from the Yitong River (Changchun district at three collection points: 1, 2 and 3); tap water from our laboratory was also tested under the same conditions. All water samples were filtered with 0.45 μm and 0.22 μm membranes. After being treated with UV digestion for release of the trace Pb2+ from Pb2+ organic complexes, the samples were analyzed by the standard addition method.3. Results and discussion
3.1 Morphology of the TCFC EP film
The morphology of the TCFC EP film was characterized by atomic force microscopy (AFM) and high resolution transmission electron microscopy (HR-TEM) as shown in Fig. 1. It can be seen from the AFM images that TCFC could form a rough film on the electrode after the EP process, and the roughness was 4.86 nm. Such morphology could improve the contact area between the EP film and Pb2+. The HR-TEM images showed the internal structure information of the EP film, which indicated that the EP film could form a cross-linking cavity microstructure, which benefits the rapid diffusion of Pb2+.3.2 Electrochemical impedance spectra and cyclic voltammograms of the different electrodes
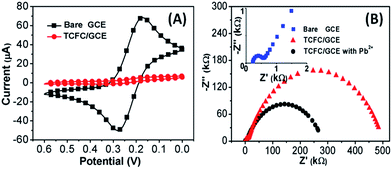
CV and EIS have been widely used for the characterization of modified electrodes, providing clear information about the electron transfer kinetics of redox probes.13 Thus, CV and EIS were performed to characterize the bare GCE, TCFC/GCE and TCFC/GCE with Pb2+. As can be seen from Fig. 2A, for the bare GCE in a solution of 5 mM Fe(CN)63−/4− in 0.1 M KCl, an obvious amperometric response could be observed. After the modification with TCFC by the EP method, the resulting TCFC/GCE presented a tremendous decrease in the amperometric response. The results indicated that TCFC had been deposited onto the GCE.The Nyquist plot of the bare GCE is shown in the inset of Fig. 2B. The diameter of the semicircle at high frequencies corresponds to the electron transfer resistance (Rct), and the linear part at low frequencies corresponds to the diffusion process.13,14 The equivalent circuit diagram can be written as:
| Rs(Q(RctW)) |
After the modification, the TCFC layer on the GCE produced an interior resistance (Ri) apart from Rct as shown in Fig. 2B. Its equivalent circuit diagram can be denoted as:
| Rs(Q1Ri)(Q2Rct) |
The Rct value for the bare GCE is 337 Ω. The Ri and Rct values for TCFC/GCE before detecting Pb2+ are 9.5 × 105 and 4.9 × 105 Ω, respectively, and those after detecting Pb2+ are 2.7 × 105 and 5051 Ω, respectively. Obviously, the Ri and Rct values for TCFC/GCE before detecting Pb2+ are larger than those after detecting Pb2+. This may be caused by the embedding of Pb2+ into the EP layer, increasing its conductivity.
3.3 Optimization of the deposition conditions
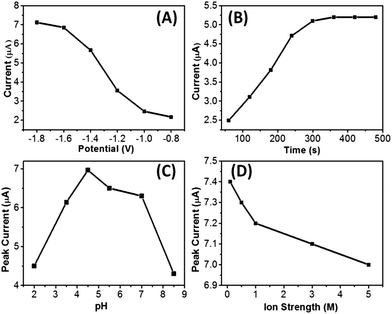
For electrochemical measurements, optimizing deposition conditions is usually a simple and effective way to enhance the sensitivity. Thus, the influence of deposition potential and deposition time on the stripping signal for Pb2+ were firstly studied, and the results are shown in Fig. 3. As can be seen from Fig. 3A, for TCFC/GCE (in 0.1 M HAc/NaAc, pH 4.5) exposed to Pb2+ (4.0 × 10−5 M), the stripping peak current of Pb2+ exhibited a notable increasing trend with a negative shift of deposition potential. The maximal peak height could be observed at −1.8 V. Considering the increasing possibility of the hydrogen evolution reaction at more negative potentials, which might damage the deposition of Pb2+ at the electrode surface, the deposition potential of −1.2 V was chosen as the optimum potential for Pb2+.The dependence on the deposition time was also investigated from 60 s to 480 s. Fig. 3B shows the relationship between the peak currents and deposition time for 4.0 × 10−5 M Pb2+ at −1.2 V in 0.1 M NaAc/HAc (pH 4.5). As can be seen in Fig. 3B, the peak currents increased with the increase of the deposition time, and after 300 s, a saturation section appeared, so a deposition time of 300 s was chosen as the optimum deposition time for the following experiments.
pH is also an important factor in HMIs sensing. Hence, we carried out an experiment to explore the influence of pH on the detection of Pb2+. As can be seen from Fig. 3C, different pHs (Na2HPO4/citric acid: pH = 2.0; NaAc/HAc: pH = 3.5, 4.5 and 5.5; PBS; pH = 7.0; and Na2CO3/NaHCO3: pH = 8.5) were investigated. Based on the achieved intensity, NaAc/HAc (pH 4.5) was selected as the optimized buffer solution.
As a parameter of pH, ion strength can influence the binding process of TCFC/GCE with Pb2+. Thus, the effect of the ion strength was also studied as shown in Fig. 3D, which indicates that the peak current decreased slowly with the increase of ion strength. From this we can easily deduce that the binding ability of TCFC towards Pb2+ decreases with the increase of ion strength. Considering this, a 0.1 M ion strength was chosen as the optimized condition.
3.4 Electrochemical detection of Pb2+
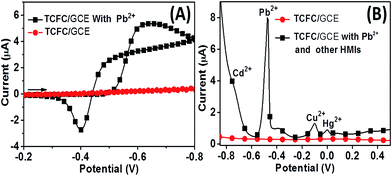
Furthermore, due to the designed metal chelating unit, PHEN, in the TCFC molecule, the chelating behavior of TCFC/GCE was also studied by CV (Fig. 4A). As can be seen, before the exposure to Pb2+, the CV of the bare TCFC/GCE was nearly a straight line (circular dots). After the accumulation of Pb2+ and rinsing with deionized water three times to remove the adsorptive Pb2+ and other electrolyte, a significant oxidation peak and reduction peak (−0.4 V and −0.65 V) appear in the CV curves, which indicate the chelation of Pb2+ to the TCFC molecule.TCFC/GCE shows excellent selectivity towards Pb2+ even in the presence of a large amount of other HMIs. Fig. 4B shows the SWASV curves of TCFC/GCE used to detect Pb2+ (1.0 × 10−8 M) with disturbing metal ions Cd2+, Cu2+ and Hg2+ (1.0 × 10−6 M). As can be seen, though the concentrations of the disturbing metal ions were 100 times higher than that of Pb2+, an intense stripping signal for Pb2+ can be clearly observed compared with the other HMIs, which indicates the high selectivity of TCFC/GCE towards Pb2+.
To ascertain the LOD value, further investigations using SWASV were carried out under the same experimental conditions as in Scheme 1. Firstly, the SWASV performance of the bare GCE with 2.0 × 10−10 M Pb2+ in NaAc/HAc buffer solution (0.1 M, pH 4.5) was measured, and no peak was observed. Then, TCFC/GCE was tested. Fig. 5 shows the SWASV of TCFC/GCE with different Pb2+ concentrations ranging from 2.0 × 10−10 M to 4.4 × 10−8 M. The inset shows an amplification of the SWASV curves for ultralow Pb2+ concentrations of 2.0 × 10−10 to 1.2 × 10−9 M. The corresponding calibration plot (Fig. 5B) revealed that the peak current increases linearly in the range of Pb2+ concentration from 2.0 × 10−10 M to 1.2 × 10−8 M (R2 = 0.9908). The final calculated LOD is 1.3 × 10−11 M, which is comparable to previous reports as shown in Table 1.13,15–21
| Electrode | Methods | Chelating unit | Analytical ranges (nM) | LOD (nM) | Matrix | Ref. |
|---|---|---|---|---|---|---|
| GCE | SWASV | Nafion/CNT/benzo-18-crown-6 | 1–30 | 1 | Tap water | 13 |
| GCE | DPASV | Thiacalixarene (TCA-MWCNT) | 0.2–10 | 0.04 | River water | 15 |
| GCE | DPV | Amino-functionalized metal–organic frameworks | 10–500 | 5 | Lake water, tap water | 16 |
| GCE | DPV | 4-Azulen-1-yl-2,6-bis(2-thie-nyl)pyridine | 1–100 | 0.7 | River water | 17 |
| GCE | SWASV | Ionophore/CNT | 1.5–250 | 0.5 | River water | 18 |
| GCE | SWASV | Nafion modified electrode | 5–100 | 5 | River water | 19 |
| GCE | DPASV | Nanomaterial/ionophore | 5–800 | 1 | Lake water | 20 |
| GPE | SWV | EDTA | 0.75–100 | 0.6 | Urine, tap water | 21 |
| GCE | SWASV | Phenanthroline (TCFC) | 0.2–12 | 0.01 | River water | This work |
3.5 Stability and recycled use of TCFC/GCE
The stability of TCFC/GCE was evaluated by repeated serial measurements each day, which were performed continuously for 30 days. To remove the chelated Pb2+ in the TCFC/GCE and gain the original SWASV window between −1.0 V to 0.4 V, the TCFC/GCE was cleaned in aqueous EDTA at a M-PS potential of 0 V for 3 min with vigorous stirring, then dried with N2 and placed in a vacuum oven at a temperature of 40 °C for 2 h, before being exposed to air for each measurement. Fig. 6 displays the SWASV responses of TCFC/GCE towards Pb2+ at a concentration of 4.4 × 10−8 M within 30 days. It can be seen that the current response of TCFC/GCE did not change considerably even after 30 days under ambient conditions. It is demonstrated that the intrinsic PHEN moiety of TCFC/GCE can repeatedly entrap Pb2+, which is of great value for practical sensors. | ||
| Fig. 6 The SWASV responses of TCFC/GCE towards Pb2+ at a concentration of 4.4 × 10−8 M within 30 days. Results are presented as mean ± SD (error bar) of triplicate experiments. | ||
Furthermore, in order to illustrate its accuracy in practical analysis, a comparison between TCFC/GCE and inductively coupled plasma mass spectrometry (ICP-MS) for Pb2+ detection in real samples (Yitong River water and tap water in our laboratory) was carried out. The results are shown in Table 2. As can be seen, for the Yitong River samples, the concentrations of Pb2+ were measured as 66.1 ± 1.5, 55.3 ± 1.8 and 23.0 ± 1.7 nM. The obtained values showed good agreement with the certified values measured by ICP-MS with good precision. TCFC/GCE was also applied successfully for the determination of Pb2+ in tap water. The concentration of Pb2+ was detected as 15.0 ± 1.5 nM. To compare the results, the tap water was also analyzed using ICP-MS. The result shows that Pb2+ was present in a concentration of 17.3 ± 1.2 nM in the tap water, indicating that TCFC/GCE can be applied to the determination of Pb2+ in real water samples.
4. Conclusions
In summary, an innovative material, TCFC, was used to modify a GCE by the EP method, and the resulting TCFC/GCE was applied to the detection of ultratrace Pb2+ with excellent performance, which might be due to the strong metal ion chelating ability of the PHEN unit in TCFC and the intrinsic cross-linking network of the EP film. TCFC/GCE showed a good linear (R2 = 0.9908) response to Pb2+ ranging from 2.0 × 10−10 to 1.2 × 10−8 M, with a calculated LOD of 1.33 × 10−11 M (S/N = 3). Moreover, TCFC/GCE is stable and can be recycled while retaining high sensitivity even after 30 days. Furthermore, the final results suggest that TCFC may be a promising competitor for detecting Pb2+ in real water samples.Acknowledgements
We are grateful for the financial support from Graduate Innovation Fund of Jilin University (Project no. 2014047), and National Science Foundation of China (no. 50973041, 21374037 and 21005008).Notes and references
- H. A. Godwin, Curr. Opin. Chem. Biol., 2001, 5, 223 CrossRef CAS.
- T. Lasantha, S. B. Viyannalage and D. Nikolay, Anal. Chem., 2008, 80, 2042 CrossRef PubMed.
- H. L. Needleman, Human Lead Exposure, CRC Press, Boca Raton, FL, 1992 Search PubMed.
- J. Wang, Analytical electrochemistry, VCH-Wiley, New York, 2nd edn, 2000 Search PubMed.
- A. J. Bard and R. L. Faulkner, Electrochemical methods: fundamentals and applications, VCH-Wiley, New York, 2nd edn, 1980 Search PubMed.
- K. Kalcher, J. M. Kauffmann, J. Wang, I. Svancara, K. Vytras, C. Neuhold and Z. Yang, Electroanalysis, 1995, 7, 5 CrossRef CAS.
- I. A. Veselova and T. N. Shekhovtsova, Anal. Chim. Acta, 2000, 413, 95 CrossRef CAS.
- W. Yantasee, C. Timchalk, K. K. Weitz, D. A. Moore and Y. Lin, Talanta, 2005, 67, 617 CrossRef CAS PubMed.
- E. Chow, D. B. Hibbert and J. J. Gooding, Anal. Chim. Acta, 2005, 543, 167 CrossRef CAS PubMed.
- X. Yi, A. A. Rowe and K. W. Plaxco, J. Am. Chem. Soc., 2007, 129, 262 CrossRef PubMed.
- C. W. Liu, C. C. Huang and H. T. Chang, Anal. Chem., 2009, 81, 2383 CrossRef CAS PubMed.
- P. Li, C. Y. Ji, H. W. Ma and M. Zhang, Chem.–Eur. J, 2014, 20, 5741 CrossRef CAS PubMed.
- S. Anandhakumar and J. Mathiyarasu, Microchim. Acta, 2013, 180, 1065 CrossRef CAS.
- R. X. Xu, X. Y. Yu, C. Gao, Y. J. Jiang, D. D. Han, J. H. Liu and X. J. Huang, Anal. Chim. Acta, 2013, 790, 31 CrossRef CAS PubMed.
- L. Wang, X. Y. Wang, G. S. Shi, C. Peng and Y. H. Ding, Anal. Chem., 2012, 84, 10560 CrossRef CAS PubMed.
- Y. Wang, H. L. Ge, Y. C. Wu, G. Q. Ye, H. H. Chen and X. Y. Hu, Talanta, 2014, 129, 100 CrossRef CAS PubMed.
- G. O. Buica, E. M. Ungureanu, L. Birzan, A. C. Razus and L. R. Mandoc (Popescu), J. Electroanal. Chem., 2013, 693, 67 CrossRef CAS PubMed.
- X. H. He, L. Chen, X. Xie, Z. H. Su, C. Qin, Y. Liu, M. Ma, S. Z. Yao, L. Deng, Q. J. Xie, Y. Tian, D. L. Qin and Y. P. Luo, Microchim. Acta, 2012, 176, 81 CrossRef CAS.
- K. Crowley and J. Cassidy, Electroanalysis, 2002, 14, 1077 CrossRef CAS.
- D. W. Pan, Y. E. Wang, Z. P. Chen, T. T. Lou and W. Qin, Anal. Chem., 2009, 81, 5088 CrossRef CAS PubMed.
- M. A. Rahman, M. S. Won and Y. B. Shim, Anal. Chem., 2003, 75, 1123 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10906f |
| This journal is © The Royal Society of Chemistry 2015 |