Solution blowing of activated carbon nanofibers for phenol adsorption
Abstract
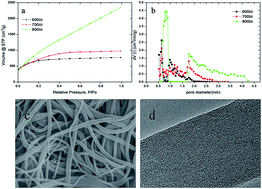
An activated carbon nanofiber (ACNF) with high surface area and excellent phenol adsorption capacity has been successfully fabricated by solution blowing of polyacrylonitrile (PAN) into precursor nanofiber which was subsequently activated to ACNF via KOH activation process. The effects of impregnation ratio and activation temperature on the texture properties of ACNF were discussed. The texture properties were characterized by N2 adsorption–desorption isotherm. ACNF showed high value of special surface area and pore volume (2921.263 m2 g−1 and 2.714 cm3 g−1, respectively). The ACNF samples were used for phenol adsorption from aqueous solutions. Adsorption isotherms at 38 °C were fitted with Langmuir and Freundlich models. The relationship between texture properties and phenol adsorption behavior was investigated. As a result, ACNF sample with large surface area, high micropore volume and pore size close and above to 0.43 nm showed the maximum phenol adsorption capacity of 251.6 mg g−1.

 Please wait while we load your content...
Please wait while we load your content...