Synthesis of vertical aligned TiO2@polyaniline core–shell nanorods for high-performance supercapacitors†
Li Zhanga,
Lei Chenb,
Bin Qia,
Guocheng Yangc and
Jian Gong*a
aKey Laboratory of Polyoxometalates Science of Ministry of Education, Northeast Normal University, Changchun, Jilin 130024, China. E-mail: gongj823@nenu.edu.cn
bSchool of Environment, Northeast Normal University, China
cSchool of Chemistry and Life Science, Changchun University of Technology, Changchun, Jilin 130012, China
First published on 21st November 2014
Abstract
Ordered arrays of a TiO2@polyaniline core–shell nanocomposite exhibiting specific capacitance as high as 820 F g−1 at 1 A g−1 were prepared by the combination of hydrothermal and in situ chemical polymerization methods. The specific capacitance retention of the nanocomposite is over 85% after 1000 charge and discharge cycles at a current density of 10 A g−1, suggesting good cycling stability.
For supercapacitors (SCs), it is important to choose a suitable electrode material with high capacity performance. As one knows, carbon materials, noble metal oxides, and conducting polymers are the three main types of greatly investigated electrode materials.1 Among them, noble metal oxides and conducting polymers have been shown to deliver higher specific capacitance than carbon materials, since they store charge through both double-layer and redox capacitive mechanisms.2–4 Compared with noble metal oxides, conducting polymers, such as polyaniline (PANI), offer more potential applications for solving the energy crisis and environmental pollution because of their high conductivity and capacitance, low cost, easy synthesis and good stability. However, as a kind of SCs material, PANI shows an obvious volume change during the charge and discharge process, which has largely decreased its mechanical stability during use.5 For the reason given above, the cycling stability is usually poor, which has become a major obstacle for PANI to be used in supercapacitors.
In order to solve the poor cyclability of PANI, a good and effective method to add inorganic support materials in PANI has been demonstrated.6–9 Among these inorganic support materials, TiO2 has been widely used and investigated because of its lower cost, environmentally friendly nature, and good adhesion between PANI and fluorine-doped tin oxide (FTO) substrate. Recently, Bian et al. reported on a TiO2/PANI fibriform composite in which the nano-TiO2 particles were embedded within the PANI fibers.10 The specific capacitance of the as-prepared composite was up to 330 F g−1 at a constant current density of 1.5 A g−1. Li et al. prepared a highly homogeneous TiO2/PANI hybrid by the oxidative polymerization of aniline with the simultaneous hydrolysis of Ti(SO4)2.11 Its initial specific capacitance is about 495 F g−1, and its capacitance retention ratio reaches 50% after 3000 consecutive cycles. Xie et al. obtained a novel composite electrode made of a PANI nanowire–titania nanotube array via electropolymerizing aniline onto an anodized titania nanotube array.12 The specific capacitance was as high as 732 F g−1 at 1 A g−1. The cycle life was maintained with a retention of 86% of the initial specific capacitance after 2000 cycles.
Following our extensive explorations of PANI materials,13–17 in this communication, we report an easy method for the synthesis of a TiO2@PANI core–shell nanocomposite with well-defined vertical aligned morphologies. An excellent specific capacitance of as high as 820 F g−1 at a charge and discharge current density of 1 A g−1 was obtained, which is the highest capacitance reported to date for TiO2@PANI materials. After 1000 cycles, the specific capacitance retention is over 85%.
A typical fabrication procedure is shown in Scheme 1. TiO2 nanorods grown on the FTO substrate were prepared first by the reported hydrothermal method.18 Then, carbon-coated TiO2 (TiO2@C) nanorods were obtained according to a similar approach to the previous report.19 For the synthesis of TiO2@C nanorods, the TiO2 nanorods on the FTO substrate were immersed into 30 mL of glucose solution (0.3 mol L−1) for 12 h. The free space between the neighbouring nanorods would allow the adsorption of glucose molecules onto the nanorod surface. After that, the FTO substrate with the TiO2 nanorods was taken out, dried and further annealed in Ar gas at 500 °C for 5 h to allow the carbonization of glucose. In the end, a layer of carbon could be painted homogenously on the surface of the TiO2 nanorods. In order to obtain the final TiO2@PANI core–shell nanorods, the TiO2@C nanorods were immersed into 12 mL of KMnO4 solution (0.03 mol L−1) and sealed in a Teflon-lined stainless steel autoclave at 160 °C for 5 h. Due to the interfacial reaction between C and KMnO4, TiO2@MnO2 core–shell nanorods were obtained. Next, 0.2 mL of aniline was added into the 30 mL of HCl solution (1 mol L−1) with stirring for 5 min. Then the FTO with TiO2@MnO2 core–shell nanorods was immersed into the above solution. The MnO2 served as the oxidant for the synthesis of PANI. After reacting for 12 h at 0–5 °C, the dark green TiO2@PANI core–shell nanorods were obtained. Detailed preparation and characterization of TiO2, TiO2@C, TiO2@MnO2, and TiO2@PANI nanorods can be found in EIS.
 | ||
| Scheme 1 Schematic illustration of the fabrication process for designed vertical aligned TiO2@PANI core–shell nanocomposite electrode. | ||
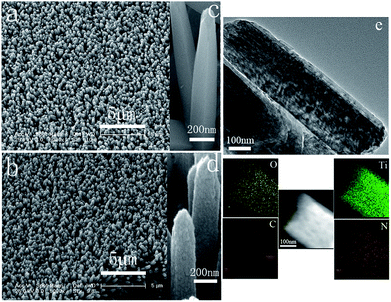
Fig. 1 shows scanning electron microscopy (SEM) images of the TiO2 nanorods (Fig. 1a and c) and the TiO2@PANI core–shell nanorods (Fig. 1b and d). The low magnification SEM image of the TiO2 nanorods (Fig. 1a) shows that the TiO2 nanorods as the core are uniform and with average diameter in the range of about 180–250 nm. The higher magnification SEM of the TiO2 nanorods (Fig. 1c) shows that the walls are relatively smooth, but the tips of them are relatively rough. After being coated with PANI shell, as shown in Fig. 1b, the TiO2@PANI nanocomposite is also uniform, and in Fig. 1d, the higher magnification SEM image of TiO2@PANI shows that it is rougher than the core TiO2, which is also supported by transmission electron microscopy (TEM) images (Fig. 1e) of the TiO2@PANI nanocomposite. A core–shell nanostructure is very obvious, and the TEM image of the TiO2@PANI core–shell nanorods shows that the thickness of the PANI shell is about 45–50 nm. The distribution of PANI on the TiO2 nanorods is investigated by STEM-EDX elemental mapping. The bottom of Fig. 1e shows that C (referring to C from PANI) is distributed similarly to Ti (referring to TiO2) and N (in PANI), O (in TiO2), indicating that a uniform PANI film is produced on the surface of TiO2. X-ray diffraction (XRD) patterns, Fourier transforms infrared spectrum (FT-IR), and energy-dispersive X-ray analysis (EDX) were also used to characterize the TiO2, TiO2@C, TiO2@MnO2 and TiO2@PANI core–shell nanorods (Fig. S1, S2 and S4 in ESI†). The morphologies of TiO2@C, TiO2@MnO2 and TiO2@PANI and pure PANI are shown in the ESI (Fig. S3).†
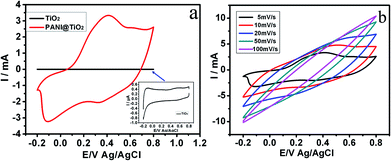
Cyclic voltammetry (CV) was used to study the electrochemical properties of nanocomposite electrode and the galvanostatic charge–discharge (GCD) test was used to demonstrate the capacitive behavior of the vertical aligned TiO2@PANI nanocomposite. In Fig. 2a, the CV curves of the vertical aligned TiO2@PANI core–shell nanorods and pure TiO2 nanorods measured at a scan rate of 5 mV s−1 are distinctly different. From the measured results, we can see that the CV curve of the TiO2@PANI core–shell nanorods shows two pairs of redox peaks of PANI. The first pair of redox peaks is attributed to the redox transition of PANI from leucoemeraldine to emeraldine states, and the other pair of peaks is ascribed to the transformation from emeraldine to pernigraniline states.20 The CV curve of the TiO2 nanorods shows a typical electric double layer capacitance. Fig. 2b shows the CV curves of the TiO2@PANI electrode at different scan rates of 5, 10, 20, 50 and 100 mV s−1. It is obvious that the shape of the CV curves does not change evidently below 20 mV s−1 and the total peak current density increases with the increase of scan rate, which demonstrates a good rate property and excellent capacitance of the TiO2@PANI electrode.21 Furthermore, the current intensity of the redox peaks increases with increasing voltage, indicating an increased resistance of the electrode materials.22
Fig. 3a shows the galvanostatic discharge (GCD) curves of the TiO2@PANI electrode at different current densities. The specific capacitance values were evaluated from discharge curves, and the detailed calculation equations about the electrodes are listed in the ESI.† The specific capacitance of the TiO2@PANI nanocomposite is as high as 820 F g−1 at a charge and discharge current density of 1 A g−1, and the corresponding energy density and power density are 102.5 W h kg−1 and 4.62 kW kg−1, respectively.
In order to improve our TiO2@PANI core–shell nanorods so that they have better electrochemical performance than other products, we conducted a comparison of TiO2@C, TiO2@MnO2, PANI and TiO2@PANI with CV and GCD test (in ESI, Fig. S5 and S6†). As a result, we confirm that the TiO2@PANI has the best performance.
Cycling stability is another important factor to determine the practical applications of SCs. The electrochemical stability of the TiO2@PANI electrode, consecutive charge–discharge cycles were measured at a current density of 10 A g−1 (Fig. 3b). The TiO2@PANI electrode was shown to keep 85% of its initial capacitance after 1000 cycle tests, suggesting that it exhibits excellent long-term cycle ability and a high degree of reversibility in consecutive charge–discharge cycles. We also provided the morphology of the TiO2@PANI core–shell nanorod (ESI Fig. S7†), and can see from it that the TiO2@PANI nanorod has not changed very obviously. Compared with the reported studies, it is obvious that the presence of well-ordered TiO2 arrays as a support for PANI is also very advantageous for reducing the electrochemical degradation of PANI and improving its cycle ability as electrode material.
To further differentiate the supercapacitor based on the vertical aligned TiO2@PANI core–shell nanorods, we tested the charge transport and ion diffusion of the composite by using electrochemical impedance spectroscopy (EIS). A Nyquist plot was generated as shown in Fig. 3c. The Nyquist plots of both the TiO2@PANI and the TiO2 nanorods consist of a semicircle in the high-to-medium frequency region and an inclined line in the low-frequency region. The semicircle corresponds to the charge-transfer impedance on the electrode/electrolyte interface, and the inclined line in the low-frequency region is assigned to the ion diffusion process within the electrodes.23 Compared with the TiO2 nanorods, the diameter of the semicircle for the vertical aligned TiO2@PANI core–shell nanorods is much smaller, revealing a greatly reduced charge-transfer resistance (Rct). The reduction of Rct should be ascribed to the large surface area and prominently improved conductivity of the vertical aligned TiO2@PANI core–shell nanorods. Moreover, in the low frequency regime, the EIS spectrum of the TiO2@PANI core–shell nanorods exhibits a more vertical straight line along the imaging axis, which indicates lower diffusion resistance in the electrode.
The more outstanding electrochemical performance of the vertical aligned TiO2@PANI core–shell nanorods can be explained. Except for the special synergistic effect of both components, the excellent performance of the nanocomposite also depends on the specific hierarchical architecture of the aligned TiO2@PANI core–shell nanorods. Firstly, vertical PANI nanorods greatly increase the specific surface area of the nanocomposite, which benefits the ion diffusion from the bulky solution to the surface of the TiO2@PANI core–shell nanorods. Therefore, the counterions can easily reach or leave the surface of the nanocomposite. Secondly, the counterions can penetrate the inner layer of the PANI and reach the surface and inner of the TiO2, realizing the efficient utilization of the electrode materials. We also provided the morphology of the TiO2@PANI core–shell nanorod after 1000 charge and discharge cycles (ESI Fig. S7†).
In summary, vertical aligned TiO2@PANI core–shell nanorods were prepared by the combination of hydrothermal and in situ chemical polymerization methods. The ordered arrays of TiO2@PANI core–shell nanorods so fabricated were found to exhibit relatively higher electrochemical capacitance. The specific capacitance was as high as 820 F g−1 at 1 A g−1 and the specific capacitance retention of the nanocomposite was over 85% after 1000 cycles of charge and discharge at a current density of 10 A g−1, suggesting good cycling stability. The good electrochemical performance was not only due to the synergistic effect of both the individual components but was also attributed to the unique vertical aligned structure of the electrode material, which provides a high surface area, a fast diffusion path for ions and long-term cycle stability. It is expected that the TiO2@PANI nanocomposite as electrode material with excellent capacitive properties will greatly promote their practical application for energy storage for supercapacitors. This study provides a facile approach to fabricate a hybrid hierarchical nanocomposite using conducting polymers and inorganic material, and also shows that the nanocomposite is suitable for application in energy storage owing to its special structure.
Acknowledgements
This work was supported by the Program for Changjiang Scholars and Innovative Research Team in University.Notes and references
- G. P. Wang, L. Zhang and J. J. Zhang, Chem. Soc. Rev., 2012, 41, 797 RSC.
- C. C. Hu, K. H. Chang, M. C. Lin and Y. T. Wu, Nano Lett., 2006, 6, 2690 CrossRef CAS PubMed.
- Z. L. Wang, R. Guo, G. R. Li, H. L. Lu, Z. Q. Liu, F. M. Xiao, M. Q. Zhang and Y. X. Tong, J. Mater. Chem., 2012, 22, 2401 RSC.
- K. R. Prasad and K. K. Miura, Chem. Mater., 2004, 16, 1845 CrossRef CAS.
- B. K. Kuila, B. Nandan, M. Bohme, A. Janke and M. Stamm, Chem. Commun., 2009, 5749 RSC.
- X. M. Feng, R. M. Li, Y. W. Ma, R. F. Chen, N. E. Shi, Q. L. Fan and W. Huang, Adv. Funct. Mater., 2011, 21, 2989 CrossRef CAS.
- Jaidev, R. I. Jafri, A. K. Mishra and S. Ramaprabhu, J. Mater. Chem., 2011, 21, 17601 RSC.
- K. Wang, Q. H. Meng, Y. J. Zhang, Z. X. Wei and M. H. Miao, Adv. Mater., 2013, 25, 1494 CrossRef CAS PubMed.
- S. H. Mujawar, S. B. Ambade, T. Battumur, R. B. Ambade and S. H. Lee, Electrochim. Acta, 2011, 56, 4462 CrossRef CAS PubMed.
- C. Q. Bian, A. Yu and H. Q. Wu, Electrochem. Commun., 2009, 11, 266 CrossRef CAS PubMed.
- X. W. Li, H. Zhang, G. C. Wang and Z. H. Jiang, J. Mater. Chem., 2010, 20, 10598 RSC.
- K. Y. Xie, J. Li, Y. Q. Lai, Z. A. Zhang, Y. X. Liu, G. G. Zhang and H. T. Huang, Nanoscale, 2011, 3, 2202 RSC.
- S. X. Yang, H. Y. Yang, H. Y. Ma, S. Guo, F. Cao, J. Gong and Y. L. Deng, Chem. Commun., 2011, 47, 2619 RSC.
- S. X. Yang, X. J. Cui, J. Gong and Y. L. Deng, Chem. Commun., 2013, 49, 4676 RSC.
- S. X. Yang, J. Gong and Y. L. Deng, J. Mater. Chem., 2012, 22, 13899 RSC.
- S. X. Yang, J. Gong and Y. L. Deng, J. Mater. Chem., 2012, 22, 24522 RSC.
- L. Zhang, H. Y. Ma, F. Cao, J. Gong and Z. M. Su, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 912 CrossRef CAS.
- B. Liu and E. S. Aydil, J. Am. Chem. Soc., 2009, 131, 3985 CrossRef CAS PubMed.
- X. Sun, Q. Li, Y. N. Lu and Y. B. Mao, Chem. Commun., 2013, 49, 4456 RSC.
- C. C. Hu and J. Y. Lin, Electrochim. Acta, 2002, 47, 4055 CrossRef CAS.
- H. S. Fan, H. Wang, N. Zhao, X. L. Zhang and J. Xu, J. Mater. Chem., 2012, 22, 2774 RSC.
- R. Montazami, V. Jain and J. R. Heflin, Electrochim. Acta, 2010, 56, 990 CrossRef CAS PubMed.
- D. D. Zhu, Y. D. Wang, G. L. Yuan and H. Xia, Chem. Commun., 2014, 50, 2876 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental and FT-IR, XRD, EDX spectra and detailed discussion. See DOI: 10.1039/c4ra10818c |
| This journal is © The Royal Society of Chemistry 2015 |