Microfluidic fabrication of chitosan microfibers with controllable internals from tubular to peapod-like structures†
Abstract
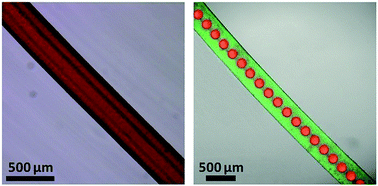
Here we report on a simple and flexible approach for continuous in situ fabrication of chitosan microfibers with controllable internals from tubular to peapod-like structures in microfluidics. Tubular and peapod-like jet templates can be generated at stable operation regions for template synthesis of chitosan microfibers with controllable tubular and peapod-like internals. The structure of each jet template can be precisely adjusted by simply changing the flow rates to tailor the structures of the resultant tubular and peapod-like chitosan microfibers. Both the tubular and peapod-like microfibers possess sufficient mechanical properties for further handling for biomedical applications. The tubular microfibers are used as biocompatible artificial vessels for transporting fluid, which is promising for delivering nutrition and blood for tissue engineering and cell culture. The peapod-like microfibers with controllable and separate oil cores can serve as multi-compartment systems for synergistic encapsulation of multiple drugs, showing great potential for developing drug-loaded medical patches for wound healing. The approach proposed in this study provides a facile and efficient strategy for controllable fabrication of microfibers with complex and well-tailored internals for biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...