Hierarchical Fe2O3@WO3 nanostructures with ultrahigh specific surface areas: microwave-assisted synthesis and enhanced H2S-sensing performance
Abstract
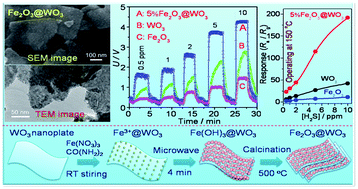
Hierarchical Fe2O3@WO3 nanocomposites with ultrahigh specific areas, consisting of Fe2O3 nanoparticles (NPs) and single-crystal WO3 nanoplates, were synthesized via a microwave-heating (MH) in situ growth process. WO3 nanoplates were derived by an intercalation and topochemical-conversion route, and the Fe2O3 NPs were in situ grown on the WO3 surfaces via a heterogamous nucleation. The water-bath-heating (WH) process was also developed to synthesize a Fe2O3@WO3 nanocomposite for comparison purposes. The techniques of X-ray diffraction (XRD), X-ray photoelectron spectrum (XPS), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to characterize the samples obtained. The results show that α-Fe2O3 NPs with a size range of 5–10 nm are uniformly, tightly anchored on the surfaces of WO3 nanoplates in the Fe2O3@WO3 samples obtained via the MH process, whereas the α-Fe2O3 NPs are not uniform in particle-sizes and spatial distribution in the Fe2O3@WO3 samples obtained via the WH process. The BET surface area of the 5wt%Fe2O3@WO3 sample derived by the MH process is as high as 1207 m2 g−1, 5.9 times higher than that (203 m2 g−1) of the corresponding WO3 nanoplates. The dramatic enhancement in the specific surface area of the Fe2O3@WO3 samples should be attributed to the hierarchical microstructure, which makes the internal surfaces or interfaces in aggregated polycrystals be fully outside surfaces via a house-of-cards configuration, where the single-layered and disconnected Fe2O3 NPs are tightly anchored on the surfaces of the WO3 nanoplates. The gas-sensing properties of the Fe2O3@WO3 sensors were investigated. The gas-sensors based on the Fe2O3@WO3 obtained via the MH process show a high response and selectivity to H2S at low operating temperatures. The 5%Fe2O3@WO3 sample shows the highest H2S-sensing response at 150 °C. Its response to 10 ppm H2S is as high as 192, 4 times higher than that of the WO3-nanoplate sensor. The improvement in the gas-sensing performance of the Fe2O3@WO3 nanocomposites can be attributed to the synergistic effect in compositions and the hierarchical microstructures with ultrahigh specific surface areas.

 Please wait while we load your content...
Please wait while we load your content...