Research progress on methane production from natural gas hydrates
Abstract
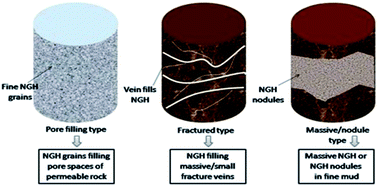
Due to the consumption of fossil fuels, an alternative energy source is necessary for the world’s continuous development. Methane hydrates, a vast energy resource that exists in deep-ocean or permafrost sediments containing approximately 10 000 Gt of carbon, are a potential energy source for the future. However, economically and safely producing methane from gas hydrate deposits is still not on the drawing board. The main reasons include (1) low methane production efficiency, (2) low methane production, (3) poor production sustainability. Thus, it is pressing to develop methane production technology and/or approaches to improve methane production efficiency. In this paper, we comprehensively review the research on methane production from gas hydrates, including the research on the characteristics of gas hydrate reservoirs, production methods, numerical simulations and field production tests. The different investigations are analyzed and relevant comments and suggestions are proposed accordingly.

 Please wait while we load your content...
Please wait while we load your content...