Fe3O4@SiO2 nanoparticles as a high-performance Fenton-like catalyst in a neutral environment†
Sheng-Tao Yang *,
Wu Zhang,
Jingru Xie,
Rong Liao,
Xiaoliang Zhang,
Baowei Yu,
Ruihan Wu,
Xiaoyang Liu,
Hongliang Li and
Zhen Guo
*,
Wu Zhang,
Jingru Xie,
Rong Liao,
Xiaoliang Zhang,
Baowei Yu,
Ruihan Wu,
Xiaoyang Liu,
Hongliang Li and
Zhen Guo
College of Chemistry and Environment Protection Engineering, Southwest University for Nationalities, Chengdu 610041, China. E-mail: yangst@pku.edu.cn
First published on 12th December 2014
Abstract
Advanced oxidation processes (AOP) have been widely applied in water treatment. However, traditional Fenton reactions based on a Fe2+–H2O2 system requires an acidic environment and generates a large amount of Fe3+ ions. Herein, we reported that magnetic Fe3O4 core–SiO2 shell nanoparticles (Fe3O4@SiO2 NPs) could be used as a Fenton-like catalyst for the decomposition of H2O2, resulting in the decoloration of methylene blue (MB). Fe3O4@SiO2 NPs had much higher activity than bare Fe3O4 cores, suggesting the coating of SiO2 enhanced the catalytic activity. Most importantly, the best performance of Fe3O4@SiO2 NPs was observed at neutral pH values. A higher temperature facilitated the diffusion of MB in solution, and thus, promoted the decoloration efficiency. The radical reaction nature was reflected by the electron spin resonance spectrum and the significant inhibition of the decoloration in the presence of the radical scavenger tertiary butanol. Fe3O4@SiO2 NPs could be magnetically separated and partially regenerated after the decoloration. The implication for the applications of Fe3O4@SiO2 NPs in water treatment is discussed.
Introduction
Water pollution is one of the most concerning environmental problems that limits the development of human society nowadays.1 Organic pollutants cause serious pollution to water environments, including dyes, antibiotics, pesticides, oil and other small organic molecules. To decontaminate the pollution, many technologies, such as advanced oxidation process (AOP), adsorption, activated sludge method and electrolysis, are developed and applied.2–5 Of particular interest and importance is the AOP method, which produces highly active radicals to oxidize the electron-enriched organic pollutants.4,5 AOP can handle diverse pollutants, especially for those not suitable for activated sludge, so AOP has been widely adopted in laboratory and industry.Fenton reaction is the most studied and used AOP method, where H2O2 is decomposed under the catalysis of Fe2+.6,7 Unfortunately, Fe2+–H2O2 system has several drawbacks that hinder its applications. The stoichiometric amounts of iron are added to the system, which consequently are oxidized into Fe3+ ions and require further treatment to get removed. The optimal condition for the production of hydroxyl radicals is pH 3, thus large quantities of acid are essential and a neutralization step is required afterward.
To these regards, heterogeneous Fenton-like catalysts based on iron or other metals are extensively researched to overcome the aforementioned drawbacks of Fe2+–H2O2 system.8–15 Many studies have demonstrated that under sonication or irradiation, Fe3O4 nanoparticles (NPs) could decontaminate many organic pollutants.16,17 Very recently, we showed that upon coating Fe3O4 NPs with a thin layer of carbon, Fe3O4 NPs catalyzed the Fenton-like reaction effectively without external energy supply.18 However, the carbon coated Fe3O4 NPs only worked well in acidic environment. Therefore, designing suitable core–shell structure of Fe3O4 NPs as Fenton-like catalyst in neutral environment becomes the major challenge.
In this study, we reported the preparation of silica coated Fe3O4 NPs (Fe3O4@SiO2 NPs) as high-performance Fenton-like catalyst in neutral environment for the decoloration of methylene blue (MB). Silica was deposited on Fe3O4 NPs by the hydrolysis of tetraethyl orthosilicate (TEOS). The decoloration efficiency of MB was measured. Influencing factors were investigated to optimize the parameters, where Fe3O4@SiO2 NPs showed even higher performance at neutral pH than under acidic conditions. The magnetic separation and regeneration were also performed. The implication to the applications of Fe3O4@SiO2 NPs as Fenton-like catalyst in water treatment is discussed.
Experimental
Materials
TEOS was bought from Jinshan Chemical Reagent Co., Ltd, China. FeCl2 was purchased from Damao Chemical Reagent Co., Ltd, China. FeCl3 was bought from Bodi Chemical Engineering Co., Ltd, China. MB was obtained from Sinopharm Chemical Reagent Co., Ltd, China. 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) was purchased from Sigma Co., America. They were used without further purification. Other chemicals were of analytical grade.Preparation of Fe3O4@SiO2 NPs
FeCl2·4H2O (0.131 g) and FeCl3·6H2O (0.269 g) were added to 50 mL water and the pH was adjusted to 12 under vigorous stirring. The mixture was further stirred for 1 h to allow the full precipitation. After the co-precipitation, Fe3O4 NPs were collected and washed with deionized water for three times. The coating of SiO2 layer was achieved by the hydrolysis of TEOS in the presence of NH3·H2O. Briefly, Fe3O4 NPs (0.1 g) were added to 1 mL of water, and then 10 mL of isopropanol was added. The mixture was sonicated for 10 min before the addition of TEOS. The mass ratios of Fe3O4 NPs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEOS were in the range of 10
TEOS were in the range of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The mixture was shaken at 100 rpm under 35 °C for 5 h. The obtained Fe3O4@SiO2 NPs were washed by water for three times and alcohol twice. The final product was dried under vacuum overnight.
10. The mixture was shaken at 100 rpm under 35 °C for 5 h. The obtained Fe3O4@SiO2 NPs were washed by water for three times and alcohol twice. The final product was dried under vacuum overnight.
Transmission electron microscopy (TEM, JEM-200CX, JEOL, Japan), X-ray photoelectron spectroscopy (XPS, Kratos, UK), Brunauer–Emmett–Teller (BET) technique (ASAP2010, Micromeritics, USA), infrared spectrometer (IR, Magna-IR 750, Nicolet, USA) and magnetometer (MPMS XL-7Tesla, Quautum Design, USA) were adopted to characterize Fe3O4@SiO2 NPs.
Decoloration of MB
To decolorize MB, 20 mL of MB (pH 6.5, 50 mg L−1) was incubated with 20 mg of Fe3O4@SiO2 NPs for 2 h (100 rpm at 35 °C) on a thermostat shaker (CHA-S, Jintan Hankang Electronic Co., China), during which the adsorption of MB reached the equilibrium. Before the addition of H2O2, 50 μL of the supernatant was collected for the absorbance (A0) measurements (664 nm) on a UV-Vis spectrometer (UV1800, PGeneral, China). Then, 1.5 mL of H2O2 was added to the mixture and incubated. At each time interval, 50 μL of the supernatant was collected for absorbance (At) measurements. The decoloration efficiency was calculated as (1 − At/A0) × 100%. The decoloration efficiency of Fe3O4@SiO2 NPs of different compositions was also measured. For comparison, the activity of Fe3O4 NPs was evaluated following the same protocol. As a control, the protocol was performed without adding catalyst. The chemical oxygen demand (COD) was determined using Hach reagent (low range 3–150 mg L−1) on Hach DR900. The kinetic constant k of decoloration was calculated following eqn (1).| −ln(C/C0) = kt | (1) |
Influencing factors
To investigate the influence of H2O2, 20 mg of Fe3O4@SiO2 NPs was used to decolorize 20 mL of MB (pH 6.5, 50 mg L−1) at 35 °C in the presence of different volumes of H2O2.To investigate the influence of catalyst amount, Fe3O4@SiO2 NPs of different amounts and 1.5 mL of H2O2 were used to decolorize 20 mL of MB (50 mg L−1) of different pH values (3.5–8.5) at 35 °C.
To investigate the influence of pH, 20 mg of Fe3O4@SiO2 NPs and 1.5 mL of H2O2 were used to decolorize 20 mL of MB (50 mg L−1) of different pH values (3.5–8.5) at 35 °C. Similarly, the influence of pH on the performance of Fe3O4 core was investigated.
To investigate the influence of temperature, 20 mg of Fe3O4@SiO2 NPs and 1.5 mL of H2O2 were used to decolorize MB (20 mL, pH 6.5, 50 mg L−1) at different temperature (0–45 °C).
To investigate the influence of inhibitor, 20 mg of Fe3O4@SiO2 NPs and 1.5 mL of H2O2 were used to decolorize 20 mL of MB (pH6.5, 50 mg L−1) at 35 °C in the presence of different amounts of tertiary butanol.
Magnetic separation and regeneration
To recycle Fe3O4@SiO2 NPs, the used Fe3O4@SiO2 NPs were magnetically separated with a magnet. The separated Fe3O4@SiO2 NPs were washed with deionized water by stirring for 20 min. After washing for three times, the recycled Fe3O4@SiO2 NPs were dried and the catalytic activity was determined following aforementioned protocol. The procedure was repeated to reach the cycles of 6. Separately, the used Fe3O4@SiO2 NPs were reduced by vitamin C (10 mg mL−1) for 2 h. After washing, the reduced Fe3O4@SiO2 NPs were subjected to activity measurement as aforementioned.Electron spin resonance (ESR) assay
For ESR assay, 100 μL sample was collected from the reaction solution (Fe3O4@SiO2 NPs/H2O2 system) at 5 min and immediately mixed with 20 μL of 0.2 mol L−1 DMPO to form DMPO–OH adduct. The ESR spectrum was obtained on a JEOLJES FA200 facility with microwave bridge (receiver gain: 1 × 105; modulation amplitude: 2 Gauss; microwave power: 4 mW; modulation frequency: 100 kHz).Results and discussion
Characterization of Fe3O4@SiO2 NPs
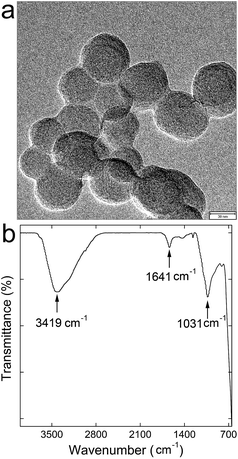
Fe3O4@SiO2 NPs were spherical particles with diameters of 20–40 nm. The core–shell structure could be roughly distinguished in Fig. 1a, where the resolution was limited by the magnetic interference to reach a clear verification of the core–shell structure. The IR spectrum in Fig. 1b was similar to those of typical SiO2 materials. The peak at 3419 cm−1 was attributed to the antisymmetric stretching vibration of –OH. The peak at 1641 cm−1 was attributed to the bending vibration of –OH. The peak at 1031 cm−1 was attributed to the antisymmetric stretching vibration of –O–Si. The chemical components were further analyzed by XPS. There were 24.5 wt% of Si and 34.6 wt% of Fe in Fe3O4@SiO2 NPs.The BET measurement indicated that the specific surface area of Fe3O4@SiO2 NPs was 124.3 m2 g−1, and the total pore volume was 0.405 cm3 g−1. The surface area of Fe3O4@SiO2 NPs was larger than that of Fe3O4 core (112.7 m2 g−1). The large surface area and the amorphous SiO2 would benefit the pre-concentration of pollutants around Fe3O4@SiO2 NPs, because amorphous SiO2 is good adsorbent for many pollutants. The N2 adsorption/desorption isotherm curve followed type IV (Fig. 2a), suggesting the nature of adsorption hysteresis. The magnetic property measurement found that the saturated magnetization was 57 emu g−1, weaker than that of bulk Fe3O4 (92.8 emu g−1),19 which could be attributed to the small size of NPs.20 The magnetic hysteresis loop indicated that Fe3O4@SiO2 NPs was ferromagnetic (Fig. 2b). The outstanding magnetic property allowed the magnetic separation of Fe3O4@SiO2 NPs after the water treatment.
Catalytic activity of Fe3O4@SiO2 NPs
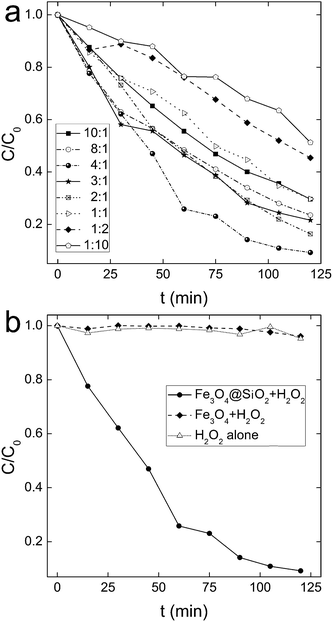
The catalytic activity of Fe3O4@SiO2 NPs was reflected by the decoloration capability of MB. We optimized the parameters of the preparation protocol. As shown in Fig. 3a, different initial ratios of TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4 led to different catalytic efficiency. The optimal ratio was 1
Fe3O4 led to different catalytic efficiency. The optimal ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, with a decoloration efficiency of 91%. The corresponding COD decreased from 101 mg L−1 to 25 mg L−1 after decoloration. The kinetic constant k at this ratio was calculated as 0.020 min−1 (Fig. S1†). This was competitive to those of the high-performance nanocatalysts in the literature.21–23 For example, Hsieh et al. reported the k value of FePt NPs–H2O2–MB system was 0.0033–0.023 min−1 at pH 5.5.21 When more TEOS was added, the decoloration efficiency decreased. A possible explanation could be that more TEOS induced a compact coating of Fe3O4 core, which hindered the diffusion of MB toward the Fe3O4 surface. Beyond that, too much SiO2 might also block the electron-transfer from iron to MB. On the other hand, when less TEOS was added, the pre-concentration effect of SiO2 shell was not dominating. Correspondingly, the promotion in catalytic activity was less.
4, with a decoloration efficiency of 91%. The corresponding COD decreased from 101 mg L−1 to 25 mg L−1 after decoloration. The kinetic constant k at this ratio was calculated as 0.020 min−1 (Fig. S1†). This was competitive to those of the high-performance nanocatalysts in the literature.21–23 For example, Hsieh et al. reported the k value of FePt NPs–H2O2–MB system was 0.0033–0.023 min−1 at pH 5.5.21 When more TEOS was added, the decoloration efficiency decreased. A possible explanation could be that more TEOS induced a compact coating of Fe3O4 core, which hindered the diffusion of MB toward the Fe3O4 surface. Beyond that, too much SiO2 might also block the electron-transfer from iron to MB. On the other hand, when less TEOS was added, the pre-concentration effect of SiO2 shell was not dominating. Correspondingly, the promotion in catalytic activity was less.
Nevertheless, coating SiO2 was very effective in enhancing the catalytic activity of Fe3O4. Although bare Fe3O4 cores had similar specific surface area, bare Fe3O4 NPs had no catalytic activity in the decomposition of H2O2 at near neutral pH (Fig. 3b). The kinetic constant k was calculated as 0.00020 min−1, suggesting the much slower decoloration kinetics (Fig. S1†). Bare Fe3O4 NPs only worked at pH 3.5 (k = 0.0046 min−1) and 8.5 (k = 0.0041 min−1) in our experiments (Fig. S2†), where the kinetics were still much lower than that of Fe3O4@SiO2–H2O2 system. The comparison clearly indicated that SiO2 coating was vital for the high performance of Fe3O4@SiO2 NPs. SiO2 at least had two important effects on Fe3O4@SiO2 NPs. Firstly, amorphous SiO2 could adsorb MB, which led to the pre-concentration of MB around Fe3O4 cores.24,25 When radicals were generated, more radicals could reach MB before the self-extinction. Another effect might be that SiO2 facilitated the electron-transfer from iron to MB.26–28 Collectively, these effects resulted in the high catalytic activity of Fe3O4@SiO2 NPs in neutral environment.
Influencing factors
We investigated the influencing factors for Fe3O4@SiO2 NPs. As indicated in Fig. 4a, more H2O2 was favorable in promoting the decoloration efficiency. Adding 1.5 mL and 2.0 mL of H2O2 showed the highest decoloration efficiency. To save H2O2, we recommended 1.5 mL H2O2. More Fe3O4@SiO2 NPs also benefited the decoloration (Fig. 4b). 20 mg of Fe3O4@SiO2 NPs showed competitive activity to 40 and 60 mg. Thus, 20 mg was used through our experiments. It was reasonable that more H2O2 and Fe3O4@SiO2 NPs promoted the decoloration from the views of kinetics and equilibrium. | ||
| Fig. 4 Influence of H2O2 (A) and Fe3O4@SiO2 NPs (B) on the decoloration of MB in Fe3O4@SiO2–H2O2 system. | ||
In the evaluations of pH, Fe3O4@SiO2 NPs showed very high activity at near neutral pH values (Fig. 5a). From pH 4.5 to pH 8.5, the decoloration efficiencies were all over 80%. At pH 6.5 the decoloration achieved 90% and the ratio was 94% at pH 7.5. The results indicated an important merit of Fe3O4@SiO2 NPs that Fe3O4@SiO2 NPs could be used in neutral environment without external energy supply.23 It was somehow surprising that Fe3O4@SiO2 NPs showed less activity at pH 3.5, which was the optimal pH for traditional Fe2+–H2O2 system. This might imply that the fundamental mechanism of the catalysis was changed. Similar phenomena were reported in literature, too.29,30 However, the mechanism of such changes still requires future investigations.
In the evaluations of temperature, the decoloration performance of Fe3O4@SiO2 NPs showed a temperature-dependent manner (Fig. 5b). Obviously, higher temperature benefited the decoloration. This was consistent with our previous observation of Fe3O4@C NPs, where higher temperature facilitated the diffusion of MB and generation of radicals.18 It should be noted that the decoloration efficiencies at 35 °C and 45 °C were very close, although the decoloration efficiency reached the maximum much faster.
The presence of radical scavenger (tertiary butanol) inhibited the decoloration. Mechanistically, the decoloration was achieved by the attack of radicals generating during the decomposition of H2O2. As shown in Fig. 6a, the ESR spectrum confirmed the presence of ˙OH radicals upon the catalysis of Fe3O4@SiO2 NPs. The ESR spectrum in the presence of Fe3O4@SiO2 NPs displayed a 4-fold characteristic peak of the typical DMPO–OH adduct with an intensity ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. When the radicals were eliminated before they reached the pollutants, the decontamination would be blocked. To this regard, we suggested that radical scavengers should be avoided during the decontamination. Again, SiO2 coating was much better than C coating.18 In our previous report, only 100 μL of tertiary butanol could completely inhibit the decoloration. In this study, the performance of Fe3O4@SiO2 NPs was only inhibited by 13%. Even when 2 mL tertiary butanol was added, the decoloration efficiency was retained as 39%. A possible explanation could be that the affinity of MB to Fe3O4@SiO2 NPs was stronger than that of tertiary butanol, which resulted in the accumulation of MB rather than tertiary butanol around Fe3O4@SiO2 NPs. Therefore, the radicals reached MB before the scavenging by tertiary butanol. Further investigations on the mechanism are highly encouraged.
1. When the radicals were eliminated before they reached the pollutants, the decontamination would be blocked. To this regard, we suggested that radical scavengers should be avoided during the decontamination. Again, SiO2 coating was much better than C coating.18 In our previous report, only 100 μL of tertiary butanol could completely inhibit the decoloration. In this study, the performance of Fe3O4@SiO2 NPs was only inhibited by 13%. Even when 2 mL tertiary butanol was added, the decoloration efficiency was retained as 39%. A possible explanation could be that the affinity of MB to Fe3O4@SiO2 NPs was stronger than that of tertiary butanol, which resulted in the accumulation of MB rather than tertiary butanol around Fe3O4@SiO2 NPs. Therefore, the radicals reached MB before the scavenging by tertiary butanol. Further investigations on the mechanism are highly encouraged.
 | ||
| Fig. 6 (A) DMPO spin-trapping ESR spectrum of ˙OH radicals in Fe3O4@SiO2–H2O2 system; (B) influence of tertiary butanol on the decoloration of MB in Fe3O4@SiO2–H2O2 system. | ||
Separation and regeneration
After the decoloration, Fe3O4@SiO2 NPs could be magnetically separated with an external magnet. The used Fe3O4@SiO2 NPs were washed with deionized water to remove most remnant MB. The regenerated Fe3O4@SiO2 NPs was used directly after the wash or dried for storage. The catalytic activity of recycled Fe3O4@SiO2 NPs was determined (Fig. 7). At cycle one, the catalytic activity of Fe3O4@SiO2 NPs was well retained without obvious loss. Thereafter, the recycling led to moderate decrease of activity of Fe3O4@SiO2 NPs. The decrease became slow when the cycle number reached 3. Despite the decrease of catalytic activity, the results indicated that Fe3O4@SiO2 NPs could be partially regenerated after the Fenton-like reaction. Thus, Fe3O4@SiO2 NPs were more environment-friendly than traditional Fe2+–H2O2 system.There might be a possibility that Fe3O4 core was oxidized during the treatment and the oxidation of Fe3O4 resulted in the activity loss. To exclude this, we reduced the recycled Fe3O4@SiO2 NPs with Na2S2O3 (Fig. 7). The reduction did not improve the regeneration, implied that the oxidation of Fe3O4 was not the main reason of the activity loss. Similar phenomena were observed when vitamin C and hydrazine hydrate were used as the reducer (data not shown). Herein, we speculated that the loss of catalytic activity might be due to the destruction of some vulnerable domains of Fe3O4@SiO2 NPs. After 3 cycles, the relative stable sites survived and the catalytic activity became almost constant.
Conclusions
In summary, Fe3O4@SiO2 NPs was prepared as high-performance Fenton-like catalyst for the decoloration of MB, where neutral pH was the optimal condition. SiO2 coating is crucial in promoting the catalytic activity and shifting the optimal pH toward neutral. Higher temperature was preferred and the radical scavengers should be avoided in the applications of Fe3O4@SiO2 NPs. The magnetic separation of Fe3O4@SiO2 NPs after the decoloration made the process environment-friendly. We hope that our results will stimulate more interests on the applications of high-performance nanocatalysts for the environmental remediation.Acknowledgements
We acknowledge financial support from Science & Technology Department of Sichuan Province (Pillar Program no. 2013FZ0060), the China Natural Science Foundation (no. 21307101), and the National Training Programs of Innovation and Entrepreneurship for Undergraduates (no. X201410656008).References
- M. G. Dosskey, Environ. Manage., 2001, 28, 577–598 CrossRef CAS PubMed.
- I. Sires and E. Brillas, Environ. Int., 2012, 40, 212–229 CrossRef CAS PubMed.
- A. Bhatnagar, F. Kaczala, W. Hogland, M. Marques, C. A. Paraskeva, V. G. Papadakis and M. Sillanpaa, Environ. Sci. Pollut. Res. Int., 2014, 21, 268–298 CrossRef CAS PubMed.
- L. Feng, E. D. van Hullebusch, M. A. Rodrigo, G. Esposito and M. A. Oturan, Chem. Eng. J., 2013, 228, 944–964 CrossRef CAS PubMed.
- J. Naumczyk, J. Bogacki, P. Marcinowski and P. Kowalik, Environ. Technol., 2014, 35, 541–548 CrossRef CAS PubMed.
- E. Neyens and J. Baeyens, J. Hazard. Mater., 2003, 98, 33–50 CrossRef CAS.
- S. Caudo, G. Centi, C. Genovese and S. Perathoner, Top. Catal., 2006, 40, 207–219 CrossRef CAS PubMed.
- N. N. Tusar, D. Maucec, M. Rangus and M. Popova, Adv. Funct. Mater., 2012, 22, 820–826 CrossRef CAS.
- S. Liu, Y. Gu, S. Wang, Y. Zhang, Y. Fang, M. D. Johnson and Y. Huang, Chin. Sci. Bull., 2013, 58, 2340–2346 CrossRef CAS.
- R. Huang, Z. Fang, X. Yan and W. Cheng, Chem. Eng. J., 2012, 197, 242–249 CrossRef CAS PubMed.
- H. Zhang, G. Zhang, X. Bi and X. Chen, J. Mater. Chem., 2013, 1, 5934–5942 RSC.
- X. Zhang, S. Gong, Y. Zhang, T. Yang, C. Wang and N. Gu, J. Mater. Chem., 2010, 20, 5110–5116 RSC.
- D. He, C. J. Miller and T. D. Waite, J. Catal., 2014, 317, 198–205 CrossRef CAS PubMed.
- M. L. Rache, A. R. Garcia, H. R. Zea, A. M. T. Silva, L. M. Madeira and J. H. Ramirez, Appl. Catal., B, 2014, 146, 192–200 CrossRef CAS PubMed.
- A. D. Bokare and W. Choi, J. Hazard. Mater., 2014, 275, 121–135 CrossRef CAS PubMed.
- R. Guo, L. Fang, W. Dong, F. Zheng and M. Shen, J. Mater. Chem., 2011, 21, 18645–18652 RSC.
- Z. Hu, B. Chen and T. Lim, RSC Adv., 2014, 4, 27820–27829 RSC.
- X. Zhang, M. He, J.-H. Liu, R. Liao, L. Zhao, J. Xie, R. Wang, S.-T. Yang, H. Wang and Y. Liu, Chin. Sci. Bull., 2014, 59, 3406–3412 CrossRef CAS.
- Z. Zhang, H. Duan, S. Li and Y. Lin, Langmuir, 2010, 26, 6676–6680 CrossRef CAS PubMed.
- C. Luna, M. P. Morales, C. J. Serna and M. Vazquez, Mater. Sci. Eng., C, 2003, 23, 1129–1132 CrossRef PubMed.
- S. Hsieh and P. Lin, J. Nanopart. Res., 2012, 14, 956 CrossRef.
- X. Hua, Y. Deng, Z. Gao, B. Liu and C. Sun, Appl. Catal., B, 2012, 127, 167–174 CrossRef PubMed.
- W. Luo, L. Zhu, N. Wang, H. Tang, M. Cao and Y. She, Environ. Sci. Technol., 2010, 44, 1786–1791 CrossRef CAS PubMed.
- Y. Xu, Y. Zhou, W. Ma, S. Wang and S. Li, J. Nanopart. Res., 2013, 15, 1716 CrossRef.
- N. Bayal and P. Jeevanandam, J. Nanopart. Res., 2013, 15, 2066 CrossRef.
- L. Xie and C. Shang, Environ. Sci. Technol., 2005, 39, 1092–1100 CrossRef CAS.
- D. Colon, E. Weber and J. Anderson, Environ. Sci. Technol., 2008, 42, 6538–6543 CrossRef CAS.
- S. Kang and W. Choi, Environ. Sci. Technol., 2009, 43, 878–883 CrossRef CAS.
- R. Huang, Z. Fang, X. Yan and W. Cheng, Chem. Eng. J., 2012, 197, 242–249 CrossRef CAS PubMed.
- J. Jiang, J. Zou, L. Zhu, L. Huang, H. Jiang and Y. Zhang, J. Nanosci. Nanotechnol., 2011, 11, 4793–4799 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: decoloration kinetics constants; catalytic performance of Fe3O4 core under different pH values. See DOI: 10.1039/c4ra10207j |
| This journal is © The Royal Society of Chemistry 2015 |