Casein glycomacropeptide hydrolysate exerts cytoprotection against H2O2-induced oxidative stress in RAW 264.7 macrophages via ROS-dependent heme oxygenase-1 expression
Xue Chengab,
Dong-Xiao Gaoa,
Jia-Jia Songa,
Fa-Zheng Rena and
Xue-Ying Mao*a
aKey Laboratory of Functional Dairy, College of Food Science and Nutritional Engineering, China Agricultural University, Beijing, P. R. China. E-mail: maoxueying@cau.edu.cn; Fax: +86 10 62738684; Tel: +86 10 62738684
bSynergetic Innovation Center of Food Safety and Nutrition, Northeast Agricultural University, Haerbin 150030, P. R. China
First published on 4th December 2014
Abstract
The aim of this study was to investigate the antioxidant potential of bovine casein glycomacropeptide (GMP) and its hydrolysate, as well as to determine effects of GMP and its hydrolysate on hydrogen peroxide (H2O2)-induced oxidative stress in RAW 264.7 macrophages. In comparison with native GMP, GMP hydrolysate obtained with papain for 1 h hydrolysis (GHP) exerted higher free radical-scavenging capacity, ferrous ion (Fe2+)-chelating ability, and ferric reducing (FRAP) activity. GHP significantly blocked H2O2-induced intracellular reactive oxygen species (ROS) generation as well as cell death, and the cytoprotective effects of GHP were partially reversed by co-treatment with zinc(II)-protoporphyrin IX (ZnPPIX), a specific inhibitor of HO-1. GHP induced nuclear translocation of the nuclear factor-erythroid-2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) expression in RAW 264.7 macrophages. The chemical antioxidant, N-acetyl cysteine (NAC), significantly reduced GHP-induced HO-1 expression and Nrf2 activation by blocking intracellular ROS production. Additionally, pretreatment with GHP enhanced cellular antioxidant enzymes activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) in H2O2-damaged cells. The antioxidant activity of GHP may be attributed to its amino acid profiles. Compared with native GMP, GHP had higher contents of alanine, glycine, glutamic acid, aspartic acid and branched chain amino acids (BCAAs, leucine, isoleucine and valine). BCAAs-enriched GHP may possess a potential to ameliorate oxidative stress-related diseases.
1. Introduction
Oxidative stress may be a hallmark of several metabolic diseases, including obesity, non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM).1–3 Oxidative stress is a condition characterized by excess production of intracellular reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide (O2−) and hydroxyl radical (˙OH) in combination with outstripping endogenous antioxidant defense systems.4,5 This oxidative stress can then damage cellular macromolecules (lipids, proteins and DNA) and triggers inflammatory responses associated with adiposity as well as insulin resistance.6–8 Inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) will in turn induce ROS generation, which leads to exacerbation of oxidative stress.9Macrophages that function as immune sentinels can release various biologically active mediators which regulate inflammation and restore tissue homeostasis.10,11 Once localized in tissues, macrophages acquire specialized functions depending on the requirements of the tissue.12 Long-term excessive ROS production caused by metabolic abnormalities will injure immunocytes, thereby resulting in cell death and loss of function. Furthermore, when continuously activated, macrophages can produce large amounts of O2− and H2O2. These ROS exert strong cytotoxic activities against many cells, including macrophages themselves.13 Excessive ROS-induced oxidative damage in macrophages which is characterized by impaired responses to tissue damage has been proposed to contribute to the pathogenesis of obesity complications such as infection and impaired tissue healing.14 Moreover, the functions of macrophages in reverse cholesterol transport can be impaired by oxidative stress.15 Protective effects against oxidative stress-associated physiological disorders were observed after systemic administration of antioxidants to mice, resulting in reduced oxidative stress in macrophages.16,17
Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) and heme oxygenase-1 (HO-1) play a key role in the defence against oxidative stress. Accumulating evidences suggest that upregulation of HO-1 expression which is mediated by activation of the nuclear factor-erythroid-2-related factor 2 (Nrf2) may confer adaptive survival response to oxidative stress both in vivo and in vitro.18,19 Antioxidant supplementation may have protective effects on oxidative damage via fortifying cellular antioxidant defense system. Lycopene inhibited the formation of ROS and affected the activities of a battery of oxidant enzymes in the skin of mice.20 Supplementation of chromium histidinate was protective against oxidative stress in obesity, at least in part, through Nrf2-mediated induction of HO-1 in rats fed high fat diet.21 Current evidences also indicate that antioxidant activity of dairy protein and derived hydrolysates. All the subunits of casein (α-casein, β-casein and κ-casein) are able to inhibit Fe-induced lipid oxidation in a liposomal model system.22 Ovine κ-casein-derived peptide was a potent inhibitor of linoleic acid oxidation with an activity similar to that obtained with the synthetic antioxidant BHT.23 Whey protein hydrolysates on H2O2-induced PC12 cells oxidative stress via a mitochondria-mediated pathway.24 Casein glycomacropeptide (GMP) is a glycopeptide of 64 amino acid residues derived from κ-casein. GMP and its hydrolysates have deserved much interest for their proposed biological activities, including antibacterial activity and antiapoptotic effect on mice with ulcerative colitis.25,26 In addition, a previous study in our laboratory indicated that GMP could counteract high-fat diet-induced obesity by reducing plasma total cholesterol and low-density lipoprotein (LDL) cholesterol as well as hepatic-cholesterol and triglycerides. Meanwhile, leptin production and pro-inflammatory cytokines such as TNF-α and IL-6 secretion also decreased.27 However, the antioxidant activity and the protective effects of GMP and its derived components on oxidative stress-induced macrophage dysfunction are still unclear. The roles of HO-1 in the protective effects of GMP and its derived components against H2O2-induced oxidative damage have not yet been elucidated.
Collectively, these data suggest that increased oxidative stress is an early instigator of metabolic syndrome and that the redox state in macrophages is a potentially useful therapeutic target for oxidative stress-related pathologies. Therefore, the aim of the present study was to evaluate the efficacy of GMP and its hydrolysates for the protection of cells against H2O2-induced oxidative damage using RAW 264.7 macrophages as a model system and to analyse the relationship between antioxidant activity and their amino acid profiles. Whether this cytoprotective effect is related to Nrf2 activation and HO-1 expression was also explored.
2. Results and discussion
2.1 Antioxidant activity of GMP and its hydrolysate
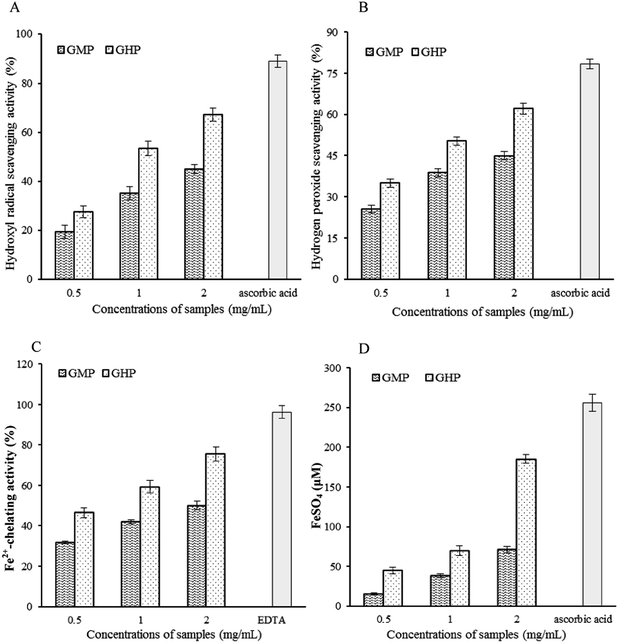
Iron toxicity has been presumed to involve the formation of OH radical from H2O2 in the Fenton reaction. H2O2 itself is not very reactive; however it can sometimes be toxic to cell because it may give rise to OH radical which is the most active ROS.28 Furthermore, H2O2 may induce the increase of iron content in cells and subsequently lead to the deleterious condition.29 Thus, minimizing Fe2+ concentration and removing free radicals may reduce the levels of ROS. Our results demonstrated that GHP showed significant OH radical scavenging activity due to its combined effects of chelating Fe2+ and scavenging H2O2. Furthermore, FRAP assay measures the reducing potential of an antioxidant reacting with a ferric tripyridyltriazine (Fe3+–TPTZ) complex and producing a coloured ferrous tripyridyltriazine (Fe2+–TPTZ). The ferric reducing antioxidant properties associated with the presence of GHP may exert antioxidant action by donating electrons through resonance to stabilize the free radicals.30 Thus, GHP may afford protection against oxidative damage by direct inhibition of ROS generation in RAW 264.7 macrophages.
2.2 Effect of GHP on H2O2-induced ROS accumulation
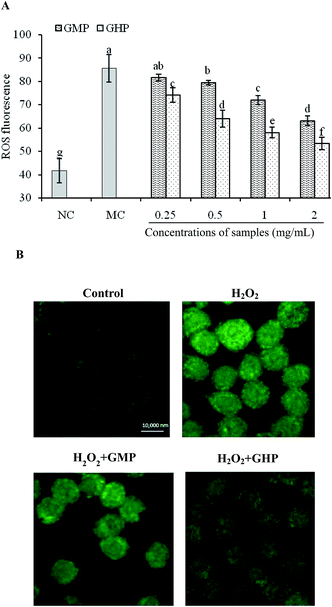
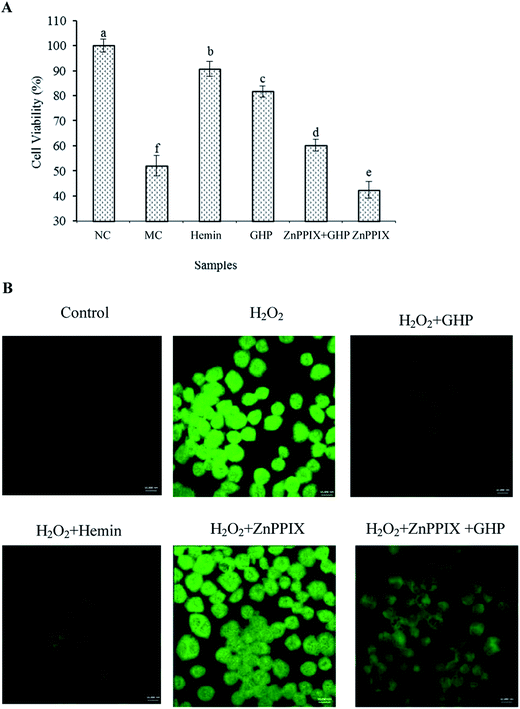
To understand whether GHP protected cellular damage via inhibiting ROS production, the effect of GHP on cellular ROS level in H2O2-stressed RAW 264.7 macrophages was assessed. The production of intracellular ROS was indirectly measured using non-fluorescent dichloro-dihydro-fluorescein diacetate (DCFH-DA), which was converted to fluorescent dichlorofluorescein (DCF) in the presence of ROS. Quantitative analysis of fluorescence intensity revealed that the intracellular ROS levels were significantly increased by H2O2 treatment (P < 0.05). GHP pretreatment significantly reduced ROS generation induced by H2O2 in a dose dependent manner from 0.25 mg mL−1 to 2.0 mg mL−1, compared with that in the H2O2-damaged group (P < 0.05), as shown in Fig. 2A. The inhibitory effect of GHP on ROS generation was significantly higher than that of GMP at each treatment concentration (P < 0.05). The intracellular ROS levels were 74.04 ± 3.18, 63.89 ± 3.66, 58.03 ± 2.25 and 53.27 ± 2.62 (decreased by 13.59%, 25.35%, 32.20% and 37.76%) at 0.25, 0.5, 1.0 and 2.0 mg mL−1 GHP, respectively. The intracellular ROS levels were 81.63 ± 1.43, 79.49 ± 0.99, 72.00 ± 1.94 and 63.12 ± 1.96 at 0.25, 0.5, 1.0 and 2.0 mg mL−1 GMP, respectively. For the intracellular ROS localization, RAW 264.7 macrophages was observed at 630× magnification under oil immersion with the laser scanning confocal microscope. As shown in Fig. 2B, the control cells displayed weak green fluorescence, whereas the cells treated with H2O2 showed striking green fluorescence, reflecting the increase of intracellular ROS levels. In contrast, this elevation was almost reversed by GHP pretreatment. These results suggested that GHP was a potential free radical scavenger.2.3 Protective effects of GHP on H2O2-induced cell death in RAW 264.7 macrophages
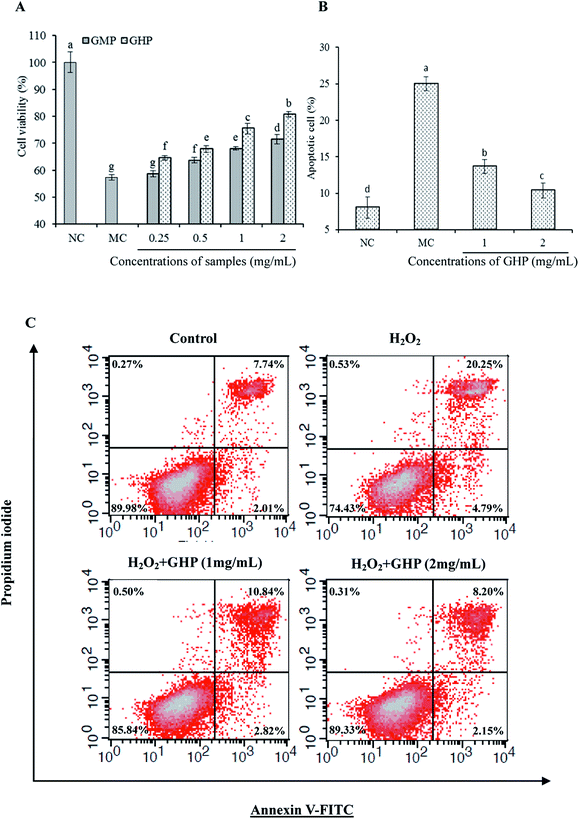
The effect of GHP on the viability of RAW 264.7 macrophages was analysed by methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay. Compared with control group (NC), the cell viability of RAW 264.7 macrophages following exposure to H2O2 (H2O2 damaged group, MC) was significantly decreased (P < 0.05), as shown in Fig. 3A. Assuming that the cell viability of control group (NC) was 100%, the cell viability was decreased to 57.26% ± 1.09% when the cells were exposed to H2O2. The cell viability of H2O2 treatment for 12 h reduced to approximately 50% of control group. The cytotoxic effects of H2O2 on RAW 264.7 macrophages were significantly blocked by pretreatment with different concentrations of GHP (P < 0.05). The protective effect of GHP on cell viability was significantly higher than that of GMP at each treatment concentration (P < 0.05). The cell viability was increased to 64.59% ± 0.86%, 67.95% ± 1.27%, 75.53% ± 1.97% and 80.70% ± 1.11% at 0.25, 0.5, 1.0 and 2.0 mg mL−1 GHP, respectively. The cell viability was increased to 58.64% ± 1.10%, 63.70% ± 1.05%, 68.01% ± 0.63% and 71.53% ± 1.69% at 0.25, 0.5, 1.0 and 2.0 mg mL−1 GMP, respectively.To investigate whether GHP protected against H2O2-induced apoptosis, RAW 264.7 macrophages were pretreated with different concentrations of GHP (1.0 and 2.0 mg mL−1) before H2O2 exposure. Cell apoptosis was examined by flow cytometry using Annexin V plus PI stain. After exposure to H2O2, the percentage of apoptotic cells increased from 8.06% ± 1.47% to 25.04% ± 0.95%, as shown in Fig. 3B. However, the percentage of apoptotic cells was decreased to 10.35% ± 1.02% by pretreatment with GHP at a concentration of 2.0 mg mL−1. Typical cytometry profiles were shown in Fig. 3C. The percentages of cells positive for PI and/or Annexin V-FITC were reported inside the quadrants.
2.4 Effect of GHP on intracellular antioxidant enzymes in RAW 264.7 macrophages subjected to H2O2-induced oxidative stress
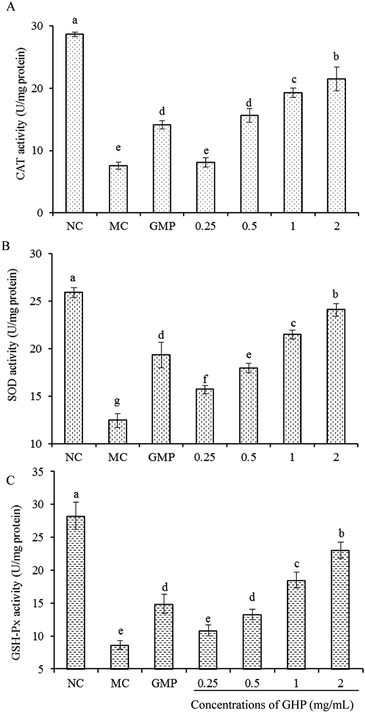
An imbalance between the antioxidant system and ROS level results in oxidative stress. To determine whether GHP can protect cellular damage by regulating intracellular antioxidant enzyme system, the effects of GHP on the activities of SOD, CAT and GSH-Px in H2O2-stressed RAW 264.7 macrophages were measured. As shown in Fig. 4, compared with normal control group (NC), the activities of SOD, CAT and GSH-Px significantly decreased in the H2O2 damaged group (MC) (P < 0.05). Upon the treatment with H2O2, the activities of SOD, CAT and GSH-Px were reduced to 12.44 ± 0.74, 7.54 ± 0.58 and 8.62 ± 0.67 U mg−1 protein, respectively. However, compared with H2O2 damaged model control, the SOD activity in response to GHP pretreatment at concentrations of 0.5, 1.0 and 2.0 mg mL−1 was increased to 17.97 ± 0.51, 21.47 ± 0.47 and 24.09 ± 0.69 U mg−1 protein (increased by 43.22%, 72.59% and 91.48%), respectively. The CAT and GSH-Px activities also significantly increased in a dose-dependent manner when H2O2-stressed RAW 264.7 macrophages were pretreated with GHP at the concentration from 0.25 mg mL−1 to 2.0 mg mL−1.Oxidative stress reflects an imbalance between the production of ROS and the removal rate of ROS by the antioxidant system, resulting in impaired cell survival thereby affecting cell functions. Macrophage dysfunction induced by oxidative stress may predispose to the development of infection and inflammatory response.31,32 Antioxidant enzymes constitute one of the major cellular protective mechanisms against oxidative injury. SOD is a potent protective enzyme that can catalyse the dismutation of O2− into H2O2 and O2. The other antioxidant enzymes including GSH-Px and CAT can catalyse the conversion of H2O2 to water.33 The present study demonstrated that GHP which exerted high antioxidant activity protected against H2O2-induced ROS generation and associated damage to cell survival. Decreased activities of CAT, SOD and GSH-Px caused by oxidative stress were also reversed by the addition of GHP. GHP may exert its protective effects against H2O2-induced oxidative damage by scavenging free radicals, Fe2+ chelation, and promoting the activity of intracellular antioxidant enzymes.
2.5 Involvement of HO-1 in cytoprotective effect of GHP
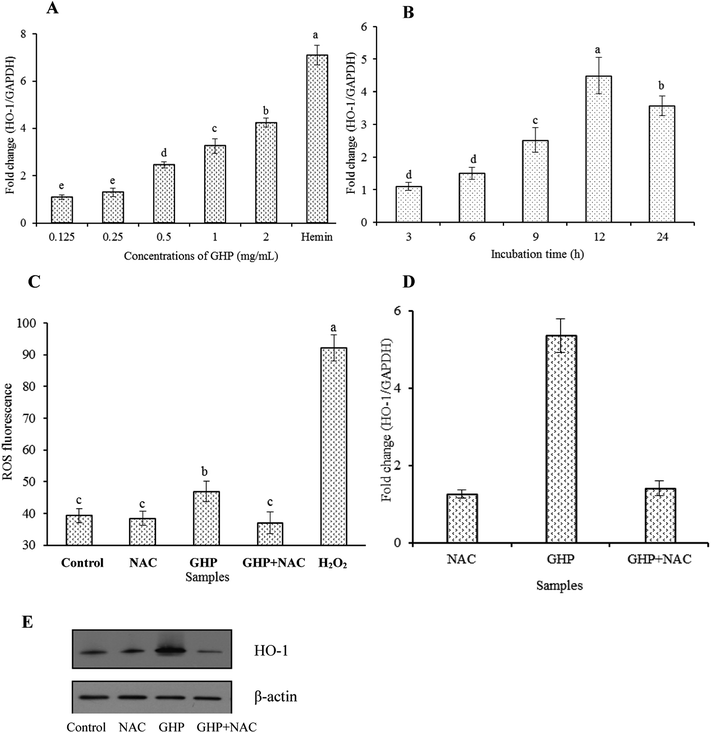
Inducible HO-1 is expressed as an adaptive response to several stimuli, including ROS, heme, cytokines and heavy metals et al. In the present culture system, a mild but significant increase in intracellular ROS levels was detected in GHP-treated RAW 264.7 macrophages. However, the addition of N-acetylcysteine (NAC), an ROS scavenger, significantly reduced intracellular ROS production induced by GHP, as shown in Fig. 6C. Strongly elevated levels of intracellular ROS in H2O2-treated macrophages was used as a positive control. Additionally, increases in HO-1 mRNA expression and protein level induced by GHP were significantly blocked by the addition of NAC, as shown in Fig. 6D and E. These data indicate that ROS induction was involved in GHP-induced HO-1 expression in RAW 264.7 macrophages.
Cells have evolved numerous pathways to counter oxidative damage including activation of stress-related protein responses. Among these, HO-1 which is an inducible stress protein provides antioxidant properties and plays an important role in protection against oxidative insult in chronic disease.34 Most importantly, HO-1 can also be induced by various antioxidants to prevent oxidative damage.35,36 Therefore, compounds that can induce HO-1 expression may be beneficial in the treatment of oxidative damage. The present study demonstrated that GHP induced HO-1 gene transcription to counteract H2O2-induced cellular injury without cytotoxicity in RAW 264.7 macrophages. While high levels of ROS production may result in oxidative stress, increasing evidence demonstrates that moderate levels of ROS may function as signaling molecules in the maintenance of physiological functions.37 Pretreatment of cells with cytoprotective agents may cause mild oxidative stresses in cells which are sufficient to initiate the intracellular signaling and consequently induce phase II enzyme genes including HO-1 to ameliorate oxidative damage.38 Epigallocatechin-3-gallate (EGCG), which possesses ROS-scavenging (antioxidant) activity, may cause mild increases in ROS production (pro-oxidant activity) in order to upregulate HO-1 expression.39 Peptides and amino acids possess both antioxidant and pro-oxidant activity, depending on certain conditions.40 In the present culture system, it was determined that GHP incubation enhanced intracellular ROS levels in accordance with stimulation of HO-1 expression, and NAC treatment inhibited GHP-induced HO-1 expression by reducing intracellular ROS production.
2.6 GHP induced Nrf2 nuclear translocation via a ROS-dependent manner in RAW 264.7 macrophages
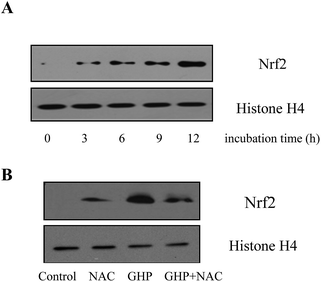
To further elucidate whether GHP induced HO-1 expression via Nrf2 activation, the nuclear level of Nrf2 protein in RAW 264.7 macrophages was analyzed by western blotting and histone H4 was used as internal control. As shown in Fig. 7A, when RAW 264.7 macrophages were incubated with GHP for 3 h, 6 h, 9 h and 12 h at the concentration of 2.0 mg mL−1, Nrf2 protein level in the nucleus significantly increased with the incubation period. GHP induced Nrf2 nuclear translocation, and thus initiated downstream gene transcription. Additionally, it was determined that GHP-induced Nrf2 nuclear translocation could be suppressed by pretreating RAW 264.7 macrophages with NAC, which suggested that ROS was essential for GHP-induced Nrf2 activation, as shown in Fig. 7B.Nrf2 is an indispensable regulator of the coordinated induction of phase II enzyme genes including HO-1. The Nrf2 is normally found in the cytoplasm bound to the inhibitory protein. On stimulation, Nrf2 translocates to the nucleus and activates target gene responsible for cytoprotection (the protection of cells from oxidative damage).41 Procyanidin B2 induces Nrf2 translocation and protects human colonic cells against oxidative stress induced by t-BOOH.42 The present results demonstrated that the nuclear level of Nrf2 protein increased with incubation time in RAW 264.7 macrophages subjected to GHP, and ROS might participate in GHP-induced Nrf2 nuclear translocation. Generally, these data imply that prevention of H2O2 induced-oxidative damage by GHP may be through its direct antioxidant activity or an indirect effect via induction of HO-1 expression by Nrf2 activation in a ROS-dependent manner.
2.7 Amino acid profiles of GMP and GHP
To further explore the reason that GHP exerts cellular antioxidant effects, the amino acid profiles of native GMP and GHP were determined, as shown in Table 1. GMP was hydrolysed by papain for 1 h and then centrifuged at 4000 g for 20 min to remove unhydrolysed protein. The contents of alanine (Ala), glycine (Gly), lysine (Lys), aspartic acid (Asp), glutamic acid (Glu) and branched chain amino acids (BCAAs) including leucine (Leu), isoleucine (Ile) and valine (Val) in GHP were higher than those in native GMP.| Amino acid | Amino acid content of GMP and GHP (μg mg−1 protein) | |
|---|---|---|
| GHP | GMP | |
| Asp | 73.83 | 52.2 |
| Thr | 125.42 | 152.37 |
| Ser | 59.59 | 62.05 |
| Glu | 169.19 | 144.29 |
| Gly | 12.94 | 7.39 |
| Ala | 79.01 | 43.82 |
| Val | 73.66 | 69.14 |
| Met | 6.04 | 14.68 |
| Ile | 88.80 | 77.41 |
| Leu | 25.44 | 12.9 |
| Phe | 8.54 | 4.71 |
| Lys | 51.25 | 43.14 |
| His | 1.48 | 2.13 |
| Arg | 3.58 | 4.13 |
| Pro | 84.04 | 91.61 |
The antioxidant activity of food proteins can be attributable to their special amino acid profiles. BCAAs, which are essential nutrients that the body obtains from proteins in food, exert an impressive protection effect against oxidative stress. Animal study has suggested supplementation with BCAAs was associated with reduced oxidative stress and improved glucose metabolism in rats with liver cirrhosis.43 In addition to BCAAs, Ala and Gly also played a protective role against oxidative stress-induced cell damage by elevating the activities of antioxidant enzymes such as HO, SOD and CAT.44,45 Pea seed protein hydrolysates with the best antioxidant properties had the highest Glu and Asp contents.46 Furthermore, the carboxyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups of Asp and Glu were often involved in chelation of toxic metals responsible for the generation of OH radical.47 Therefore, it is believed that peptides with high contents of these amino acids can enhance cellular antioxidant activity. The data in the present study demonstrated that GHP enriched with BCAAs, Ala, Asp, Glu and Gly protected macrophages from oxidative damage by scavenging ROS and activating antioxidant mechanisms.
O) groups of Asp and Glu were often involved in chelation of toxic metals responsible for the generation of OH radical.47 Therefore, it is believed that peptides with high contents of these amino acids can enhance cellular antioxidant activity. The data in the present study demonstrated that GHP enriched with BCAAs, Ala, Asp, Glu and Gly protected macrophages from oxidative damage by scavenging ROS and activating antioxidant mechanisms.
3. Experimental
3.1 Materials
GMP (GMP content of protein is minimum 95%) was provided by Arla Co. (Sønderhøj, Denmark). Papain (EC 3.4.22.21, with activity of 2 U mg−1), methylthiazolyldiphenyl-tetrazolium bromide (MTT), dimethyl sulphoxide (DMSO), guanidine hydrochloride, dichloro-dihydro-fluorescein diacetate (DCFH-DA), N-acetylcysteine (NAC), hemin and zinc(II)-protoporphyrin IX (ZnPPIX) were obtained from Sigma-Aldrich (St Louis, MO, USA). The bicinchoninic acid (BCA) protein assay kit was obtained from Pierce (Rockford, IL, USA). Primary rabbit monoclonal HO-1 and Nrf2 antibodies were purchased from Cell Signaling Technology (CST, Beverly, MA). Anti-actin antibody, anti-histone H4 antibody and horseradish peroxidase-conjugated anti-species (mouse and rabbit) secondary antibodies were purchased from Beyotime Biotech (Haimen, Jiangsu, China).3.2 Preparation of GMP hydrolysate (GHP)
GMP was dissolved in distilled water at a concentration of 5% (w/v). The protein solutions were adjusted to the optimum pH and temperature for the enzymatic hydrolysis process. Afterward, the protein solution was hydrolysed by papain for 1 h at an enzyme to protein ratio of 5% (w/w, defined as enzyme mass/substrate mass × 100%) under the optimal condition (pH 6.0, 55 °C) as recommended by the manufacturer. During the hydrolysis process, 1 M NaOH solution was continuously added to maintain the optimal pH. After hydrolysis, GMP hydrolysate were collected and immediately heated at 85 °C in a water bath for 20 min to inactivate the protease and stop the hydrolysis reaction. When they were cooled to room temperature, the hydrolysate solutions were centrifuged at 4000g for 20 min to remove unhydrolysed protein. The supernatants were collected and lyophilized.3.3 Determination of antioxidant activity of GMP and GHP
| Hydroxyl radical scavenging rate (%) = (AS − AP)/(AB − AP) × 100 |
| Hydrogen peroxide scavenging rate (%) = (A0 − A1)/A0 × 100 |
| Fe2+-chelating activity (%) = (A0 − A1)/A0 × 100 |
3.4 Cell viability analysis
Murine macrophage-like RAW 264.7 cells were obtained from American Type Culture Collection (Manassas, VA, USA). They were cultured at 37 °C with 5% carbon dioxide (CO2) in Dulbecco's Modified Eagle's Medium (DMEM, Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (FBS, Bioin, Israel), 100 U mL−1 penicillin and 100 μg mL−1 streptomycin (Invitrogen, Carlsbad, CA, USA). H2O2 was freshly prepared from 30% stock solution prior to each experiment.Cell survival was measured by MTT assay on the basis of the succinate dehydrogenase mitochondrial activity. In brief, RAW 264.7 macrophages were seeded onto 96-well plates at the density of 5 × 104 cells per well and grown to reach 50–60% confluence. Tested samples were diluted in K/Na phosphate buffer (PBS, pH 7.4). The cells were then preincubated with different concentrations of tested samples (0.25, 0.5, 1.0, 2.0 mg mL−1, final concentration in cell plate) for 12 h before exposure to H2O2 (250 μM, 12 h). Oxidatively stressed cells in the presence or absence of tested samples were subsequently treated with MTT solution (5 mg mL−1). After 4 h of incubation at 37 °C, the reaction was terminated and the formed formazan was solubilized in DMSO (150 μL). The absorbance of each well was measured at 570 nm with a microplate reader (Bio-Rad, Hercules, CA, USA). Cells treated with PBS alone were set as the control group. Cell viability (%) was expressed as the optical density ratio of each treatment group to the control group. Viability of the control group (PBS only) was taken as 100%.
3.5 Annexin V-FITC/PI apoptosis assay
The cell apoptosis rate was evaluated by Annexin V-FITC apoptosis detection kit (eBioscience, San Diego, CA, USA). Briefly, the cultured RAW 264.7 macrophages were harvested by trypsinization and resuspended in 1× Annexin V binding buffer at a concentration of 106 cells mL−1. The cells were then stained with 5 μL Annexin V-FITC and 5 μL PI in the dark and subsequently analysed with flow cytometer (BD Bioscience, San Jose, CA, USA).3.6 Cellular reactive oxygen determination
Intracellular ROS level was assessed using DCFH-DA as fluorescent label. RAW 264.7 macrophages were seeded at a density of 2 × 105 cells per well in a 96-well culture plate. The cells were treated with H2O2 after being pretreated with or without tested samples for 12 h. After incubation, the cells were washed with phosphate buffered saline (PBS, pH 7.4). DCFH-DA (final concentration 50 μM) diluted in DMEM without phenol red was added to the cells and then incubated for 60 min at 37 °C. Subsequently, the cells were washed three times with PBS to remove extracellular DCFH-DA, and then subjected to fluorescence intensity analysis using a fluorescence plate reader (Fluoroskan Ascent, Thermo Electron Corporation, Milford, MA, USA) at wavelengths of 488 nm for excitation and 520 nm for emission. The intracellular DCF fluorescence in cell samples relative to unstained cells indicated the relative levels of ROS present in the cells. ROS production was imaged using laser scanning confocal microscopy (Zeiss LSM780, Oberkochen, Germany).3.7 Analysis of cellular antioxidant enzymes activity
SOD, CAT and GSH-Px activity were determined using the commercial assay kits (Jiancheng Biochemical, Inc., Nanjing, China). In brief, superoxide anion can convert a tetrazolium salt to a formazan dye. Addition of SOD to this reaction reduced O2− levels, thereby lowering the rate of formazan dye formation. SOD activity was measured as the percent inhibition of the rate of formazan dye formation. One unit of SOD was defined as the amount of SOD required for 50% inhibition of the formazan formation. CAT activity was determined based on alteration of H2O2 optical density, depending on enzymatic decomposition of H2O2 (by the effect of CAT in the sample). One unit of CAT was defined as the decomposition of 1 μM H2O2 per second. GSH-Px activity was determined by the reduction of GSSG formed via the NADPH–glutathione reductase system as a continuous indicator system. Loss of NADPH was monitored continuously at 340 nm. The activity of GSH-Px was defined as the decrease of 1 μM GSH per minute and mg protein. The activities of enzymes were expressed as U mg−1 protein of the sample.3.8 Real time quantitative polymerase chain reaction (RT-qPCR)
RAW 264.7 macrophages (1 × 106 cells) were incubated with tested samples or PBS at 37 °C. Total cellular RNA was isolated using Trizol reagent (Tiangen Biotech, Beijing, China). The total RNA (3 μg) was converted to cDNA in a 25 μL reaction volume using TIANScript RT Kit (Tiangen Biotech), according to the manufacturer's instructions. Quantitative RT-PCR was carried out with a Techne Quantica real-time PCR detection system (Techne, Staffordshire, UK) using a SYBR Premix Ex Taq RT-PCR kit (Takara, Otsu, Shiga, Japan) to detect double stranded DNA synthesis. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was served as the internal control. Primer sequences used for amplifications were as follows: 5′-CAGAAGAGGCTAAGACCGCCTT-3′ (sense) and 5′-TCTGGTCTTTGTGTTCCTCTGTCA-3′ (antisense) for heme oxygenase-1 (HO-1); 5′-TGGCAAAGTGGAGATTGTTGC-3′ (sense) and 5′-AAGATGGTGATGGGCTTCCCG-3′ (antisense) for GAPDH. Real time quantitative PCR was performed starting with a denaturation at 95 °C for 120 s followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s, 72 °C for 30 s. The SYBR green fluorescence was read at the end of each extension step (72 °C). The specificity of the amplified PCR products was assessed by a melting curve analysis. Analysis of the data was performed by the ΔΔCt method using GAPDH for normalisation of the samples.51 The results were presented as fold change relative to the control cells.3.9 Western blotting
The cells were harvested with cell lysis buffer (Beyotime, Haimen, Jiangsu, China) containing 1 mM phenylmethylsulfonyl fluoride (PMSF, Sigma-Aldrich). Equal amounts of protein samples were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). These membranes were blocked with 5% skim milk in PBS-T solution (0.05% Tween-20 in 1 × PBS solution) at room temperature for 2 h and subsequently incubated overnight with the appropriate primary antibody at 4 °C. After washing with PBS-T solution, the PVDF membranes were incubated with peroxidase conjugated secondary antibody for 1 h. Immunolabeled proteins were detected using an immobilon western chemiluminescent HRP substrate (ECL, Millipore).3.10 Amino acid analysis
The amino acid compositions of the tested samples were analyzed by the method of Bidlingmeyer et al.52 The samples were incubated with 6 N HCl and heated at 110 °C for 24 h in oil bath. Internal standard was added to the mixture. After derivatisation with phenylisothiocyanate (PITC), the PITC-amino acids were identified and quantified by reversed-phase high-performance liquid chromatography (RP-HPLC; Shimadzu Co., Tokyo, Japan).3.11 Statistical analysis
The data were expressed as means ± standard deviations (SD). The differences among the groups were analysed by one-way analysis variance (ANOVA) followed by Tukey's method. P < 0.05 was considered statistically significant.4. Conclusion
This work demonstrated that GHP could attenuate H2O2-induced oxidative stress injury in macrophages. The protective effects of GHP partly depended on the combination of alleviating intracellular ROS production and restoring the activities of endogenous antioxidants. GHP ameliorated H2O2-oxidative damage by induction of HO-1 expression and Nrf2 activation via a ROS dependent manner. Furthermore, the antioxidant effects of GHP had a great relationship with the content of BCAAs, Ala, Gly, Lys, Asp and Glu. Therefore, GHP has the potential to be developed as a nutraceutical antioxidant for health promotion and prevention of oxidative stress-induced diseases.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant no. 31371753) and the Graduate Research and Innovation Projects of China Agricultural University (Grant no. 2012YJ077).References
- D. P. Schuster, Obesity and the development of type 2 diabetes: the effects of fatty tissue inflammation, Diabetes, Metab. Syndr. Obes.: Targets Ther., 2010, 3, 253–262 CrossRef CAS.
- L. A. Videla, R. Rodrigo, M. Orellana, V. Fernandez, G. Tapia, L. Quinones, N. Varela, J. Contreras, R. Lazarte, A. Csendes, J. Rojas, F. Maluenda, P. Burdiles, J. C. Diaz, G. Smok, L. Thielemann and J. Poniachik, Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients, Clin. Sci., 2004, 106, 261–268 CrossRef CAS PubMed.
- F. Folli, D. Corradi, P. Fanti, A. Davalli, A. Paez, A. Giaccari, C. Perego and G. Muscogiuri, The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach, Curr. Diabetes Rev., 2011, 7, 313–324 CrossRef CAS.
- K. Apel and H. Hirt, Reactive oxygen species: metabolism, oxidative stress, and signal transduction, Annu. Rev. Plant Biol., 2004, 55, 373–399 CrossRef CAS PubMed.
- J. M. Li and A. M. Shah, ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy, J. Am. Soc. Nephrol., 2003, 14(Suppl. 3), 221–226 CrossRef.
- B. Frei, Reactive oxygen species and antioxidant vitamins: mechanisms of action, Am. J. Med., 1994, 97(Suppl. 1), 5–13 CrossRef.
- J. B. Meigs, M. G. Larson, C. S. Fox, J. F. Keaney, R. S. Vasan and E. J. Benjamin, Association of oxidative stress, insulin resistance, and diabetes risk phenotypes the Framingham offspring study, Diabetes Care, 2007, 30, 2529–2535 CrossRef CAS PubMed.
- M. Krzystek-Korpacka, E. Patryn, D. Boehm, I. Berdowska, B. Zielinski and A. Noczynska, Advanced oxidation protein products (AOPPs) in juvenile overweight and obesity prior to and following weight reduction, Clin. Biochem., 2008, 41, 943–949 CrossRef CAS PubMed.
- A. Fernández-Sánchez, E. Madrigal-Santillán, M. Bautista, J. Esquivel-Soto, A. Morales-González, C. Esquivel-Chirino, I. Durante-Montiel, G. Sánchez-Rivera, C. Valadez-Vega and J. A. Morales-González, Inflammation, oxidative stress, and obesity, Int. J. Mol. Sci., 2011, 12, 3117–3132 CrossRef PubMed.
- D. L. Laskin, Macrophages and inflammatory mediators in chemical toxicity: a battle of forces, Chem. Res. Toxicol., 2009, 22, 1376–1385 CrossRef CAS PubMed.
- B. K. Surmi and A. H. Hasty, Macrophage infiltration into adipose tissue: initiation, propagation and remodeling, Future Lipidol., 2008, 3, 545–556 CrossRef CAS PubMed.
- D. L. Laskin, V. R. Sunil, C. R. Gardner and J. D. Laskin, Macrophages and tissue injury: agents of defense or destruction, Annu. Rev. Pharmacol. Toxicol., 2011, 51, 267–288 CrossRef CAS PubMed.
- J. E. Albina, S. Cui, R. B. Mateo and J. S. Reichner, Nitric oxide-mediated apoptosis in murine peritoneal macrophages, J. Immunol., 1993, 150, 5080–5085 CAS.
- J. D. Schilling, H. M. Machkovech, L. He, A. Diwan and J. E. Schaffer, TLR4 activation under lipotoxic conditions leads to synergistic macrophage cell death through a TRIF-dependent pathway, J. Immunol., 2013, 190, 1285–1296 CrossRef CAS PubMed.
- W. Korytowski, K. Wawak, P. Pabisz, J. C. Schmitt and A. W. Girotti, Macrophage mitochondrial damage from StAR transport of 7-hydroperoxycholesterol: Implications for oxidative stress-impaired reverse cholesterol transport, FEBS Lett., 2014, 588, 65–70 CrossRef CAS PubMed.
- L. Fakhrzadeh, J. D. Laskin, C. R. Gardner and D. L. Laskin, Superoxide dismutase-overexpressing mice are resistant to ozone-induced tissue injury and increases in nitric oxide and tumor necrosis factor-α, Am. J. Respir. Cell Mol. Biol., 2004, 30, 280–287 CrossRef CAS PubMed.
- V. M. Victor, M. Rocha and M. De la Fuente, Regulation of macrophage function by the antioxidant N-acetylcysteine in mouse-oxidative stress by endotoxin, Int. Immunopharmacol., 2003, 3, 97–106 CrossRef CAS.
- P. Yao, A. Nussler, L. Liu, L. Hao, F. Song, A. Schirmeier and N. Nussler, Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways, J. Hepatol., 2007, 47, 253–261 CrossRef CAS PubMed.
- C. Yang, X. Zhang, H. Fan and Y. Liu, Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia, Brain Res., 2009, 1282, 133–141 CrossRef CAS PubMed.
- C. Shen, S. Wang, Y. Shan, Z. Liu, F. Fan, L. Tao, Y. Liu, L. Zhou, C. Pei, H. Wu, C. Tian, J. Ruan, W. Chen, A. Wang, S. Zheng and Y. Lu, Chemomodulatory efficacy of lycopene on antioxidant enzymes and carcinogen-induced cutaneum carcinoma in mice, Food Funct., 2014, 5, 1422–1431 CAS.
- M. Tuzcu, N. Sahin, C. Orhan, C. A. Agca, F. Akdemir, Z. Tuzcu, J. Komorowski and K. Sahin, Impact of chromium histidinate on high fat diet induced obesity in rats, Nutr. Metab., 2011, 8, 28 CAS.
- G. Cervato, R. Cazzola and B. Cestaro, Studies on the antioxidant activity of milk casein, Int. J. Food Sci. Nutr., 1999, 50, 291–296 CrossRef CAS.
- J. Á. Gómez-Ruiz, I. López-Expósito, A. Pihlanto, M. Ramos and I. Recio, Antioxidant activity of ovine casein hydrolysates: identification of active peptides by HPLC–MS/MS, Eur. Food Res. Technol., 2008, 227, 1061–1067 CrossRef.
- M. M. Jin, L. Zhang, H. X. Yu, J. Meng, Z. Sun and R. R. Lu, Protective effect of whey protein hydrolysates on H2O2-induced PC12 cells oxidative stress via a mitochondria-mediated pathway, Food Chem., 2013, 141, 847–852 CrossRef CAS PubMed.
- Q. S. Chen, H. Wang, C. Zhu, Y. L. Yan and G. C. Pang, Anti-apoptotic effects of milk-derived casein glycomacropeptide on mice with ulcerative colitis, Food Agric. Immunol., 2014, 25, 453–466 CrossRef CAS.
- G. Robitaille, C. Lapointe, D. Leclerc and M. Britten, Effect of pepsin-treated bovine and goat caseinomacropeptide on Escherichia coli and Lactobacillus rhamnosus in acidic conditions, J. Dairy Sci., 2012, 95, 1–8 CrossRef CAS PubMed.
- S. P. Xu, X. Y. Mao, X. Cheng and B. Chen, Ameliorating effects of casein glycomacropeptide on obesity induced by high-fat diet in male Sprague–Dawley rats, Food Chem. Toxicol., 2013, 56, 1–7 CrossRef CAS PubMed.
- C. C. Winterbourn, Toxicity of iron and hydrogen peroxide: the Fenton reaction, Toxicol. Lett., 1995, 969–974 CrossRef CAS.
- J. H. Yoon, S. H. An, I. G. Kyeong, M. S. Lee, S. C. Kwon and J. H. Kang, Oxidative modification of ferritin induced by hydrogen peroxide, BMB Rep., 2011, 44, 165–169 CrossRef CAS PubMed.
- S. Dudonne, X. Vitrac, P. Coutiere, M. Woillez and J. M. Mérillon, Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays, J. Agric. Food Chem., 2009, 57, 1768–1774 CrossRef CAS PubMed.
- P. Bandaru, H. Rajkumar and G. Nappanveettil, The impact of obesity on immune response to infection and vaccine: an insight into plausible mechanisms, Endocrinology & Metabolic Syndrome, 2013, 2, 113–121 Search PubMed.
- P. Kirkham, Oxidative stress and macrophage function: a failure to resolve the inflammatory response, Biochem. Soc. Trans., 2007, 35, 284–287 CrossRef CAS PubMed.
- B. Halliwell, Free radicals and antioxidants: a personal view, Nutr. Rev., 1994, 52, 253–265 CrossRef CAS PubMed.
- B. G. Park, C. I. Yoo, H. T. Kim, C. H. Kwon and Y. K. Kim, Role of mitogen-activated protein kinases in hydrogen peroxide-induced cell death in osteoblastic cells, Toxicology, 2005, 215, 115–125 CrossRef CAS PubMed.
- N. G. Abraham and A. Kappas, Pharmacological and clinical aspects of heme oxygenase, Pharmacol. Rev., 2008, 60, 79–127 CrossRef CAS PubMed.
- M. C. Chen, Y. Y. Ye, G. Ji and J. W. Liu, Hesperidin upregulates heme oxygenase-1 to attenuate hydrogen peroxide-induced cell damage in hepatic L02 cells, J. Agric. Food Chem., 2010, 58, 3330–3335 CrossRef CAS PubMed.
- M. Schieber and N. S. Chandel, ROS Function in Redox Signaling and Oxidative Stress, Curr. Biol., 2014, 24, R453–R462 CrossRef CAS PubMed.
- K. Min, J. T. Lee, E. Joe and T. K. Kwon, An IκBα phosphorylation inhibitor induces heme oxygenase-1 (HO-1) expression through the activation of reactive oxygen species (ROS)–Nrf2–ARE signaling and ROS–PI3K/Akt signaling in an NF-κB-independent mechanism, Cell. Signalling, 2011, 23, 1505–1513 CrossRef CAS PubMed.
- C. C. Wu, M. C. Hsu, C. W. Hsieh, J. B. Lin, P. H. Lai and B. S. Wung, Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways, Life Sci., 2006, 78, 2889–2897 CrossRef CAS PubMed.
- F. Shahidi, in Natural antioxidants: chemistry, health effects, and applications, ed. P. J. White and Y. Xing, The American Oil Chemists Society, 1st edn, 1996, ch. 3, p. 32 Search PubMed.
- H. Motohashi and M. Yamamoto, Nrf2-Keap1 defines a physiologically important stress response mechanism, Trends Mol. Med., 2004, 10, 549–557 CrossRef CAS PubMed.
- I. Rodríguez-Ramiro, S. Ramos, L. Bravo, L. Goya and M. Á. Martín, Procyanidin B2 induces Nrf2 translocation and glutathione S-transferase P1 expression via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress, Eur. J. Nutr., 2012, 51, 881–892 CrossRef PubMed.
- M. Iwasa, Y. Kobayashi, R. Mifuji-Moroka, N. Hara, H. Miyachi, R. Sugimoto, H. Tanaka, N. Fujita, E. C. Gabazza and Y. Takei, Branched-chain amino acid supplementation reduces oxidative stress and prolongs survival in rats with advanced liver cirrhosis, PLoS One, 2013, 8, e70309 CAS.
- N. Grosser, S. Oberle, G. Berndt, K. Erdmann, A. Hemmerle and H. Schröder, Antioxidant action of L-alanine: heme oxygenase-1 and ferritin as possible mediators, Biochem. Biophys. Res. Commun., 2004, 314, 351–355 CrossRef CAS PubMed.
- R. Selvaraju and K. Subbashinidevi, Impact of glycine on antioxidant defence system in rats with alcohol induced liver injury, Int. J. Pharm. Biomed. Res., 2011, 2, 1314–1320 Search PubMed.
- T. L. Pownall, C. C. Udenigwe and R. E. Aluko, Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions, J. Agric. Food Chem., 2010, 58, 4712–4718 CrossRef CAS PubMed.
- T. Dudev and C. Lim, Effect of carboxylate-binding mode on metal binding/selectivity and function in proteins, Acc. Chem. Res., 2007, 40, 85–93 CrossRef CAS PubMed.
- W. L. Yu, Y. P. Zhao, Z. Xue, H. Jin and D. P. Wang, The antioxidant properties of lycopene concentrate extracted from tomato paste, J. Am. Oil Chem. Soc., 2001, 78, 697–701 CrossRef CAS PubMed.
- G. C. Yen and D. Y. Chung, Antioxidant effects of extracts from Cassia tora L. Prepared under different degrees of roasting on the oxidative damage to biomolecules, J. Agric. Food Chem., 1999, 47, 1326–1332 CrossRef CAS PubMed.
- P. Carter, Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine), Anal. Biochem., 1971, 40, 450–458 CrossRef CAS.
- M. W. Pfaffl, A new mathematical model for relative quantification in real-time RT-PCR, Nucleic Acids Res., 2001, 29, 2002–2007 CrossRef PubMed.
- B. A. Bidlingmeyer, S. A. Cohen, T. L. Tarvin and B. Frost, A new, rapid, high-sensitivity analysis of amino acids in food type samples, J. Assoc. Off. Anal. Chem., 1986, 70, 241–247 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |