Cobalt phthalocyanine tetracarboxylic acid modified reduced graphene oxide: a sensitive matrix for the electrocatalytic detection of peroxynitrite and hydrogen peroxide
Abstract
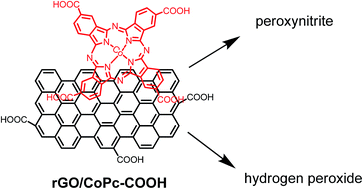
The quantification of peroxynitrite (ONOO−, PON) and hydrogen peroxide (H2O2) is intrinsically difficult as both species show similar oxidative features located within a narrow potential. The sub-second lifetime of ONOO− at neutral pH further complicates the analysis. In this paper, we examine the electrocatalytic activity of cobalt phthalocyanine tetracarboxylic acid (CoPc–COOH) loaded reduced graphene oxide (rGO) films towards peroxynitrite and hydrogen peroxide detection. The rGO/CoPc–COOH matrix is synthesized by the reaction of graphene oxide (GO) and CoPc–COOH at 90 °C for 5 h under ultrasonication. The integration of CoPc–COOH and the reduction of GO to rGO was confirmed by X-ray photoelectron spectroscopy, FTIR, Raman, UV-vis spectroscopy and electrochemistry. The rGO/CoPc–COOH film showed high electrocatalytic activity and specificity for ONOO− at anodic potential with a sensitivity of ≈11.5 ± 1 nA nM−1 and a peroxynitrite detection limit of ≈1.7 nM. The rGO/CoPc–COOH films further exhibited electrocatalytic reduction of H2O2 with a sensitivity of 14.5 μA mM−1 and a detection limit of ≈60 μM for H2O2.
- This article is part of the themed collection: Nanoscience and nanotechnology in electrochemistry

 Please wait while we load your content...
Please wait while we load your content...