A highly selective and sensitive fluorescence “turn-on” fluoride ion sensor†
Qi Lin*,
Qing-Ping Yang,
Bin Sun,
Jin-Chao Lou,
Tai-Bao Wei* and
You-Ming Zhang*
Key Laboratory of Eco-Environment-Related Polymer Materials, Ministry of Education of China, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu 730070, P. R. China. E-mail: linqi2004@126.com; weitaibao@126.com
First published on 7th January 2015
Abstract
A colorimetric and turn-on fluorescent sensor for fluoride ions, based on 2-hydroxy-1-naphthalene formaldehyde bis-Schiff base, was readily synthesized. The sensor in DMSO exhibits high sensitivity and good selectivity for fluoride ions from aqueous media. The F−-response mechanism involves a hydrogen bonding and deprotonation process in the sensor, which induced color changes from pale yellow to deep yellow and prominent fluorescence enhancement. The response time of sensor S for fluoride ions reached the plateau region after less than 1 s. Test strips based on sensor S were prepared, the test strips could conveniently detect fluoride ions in solutions. The detection limit was determined to be 1.4 × 10−8 mol L−1 (2.66 × 10−4 ppm), which is far lower than the WHO guideline for drinking water at levels of about (5.3–7.9) × 10−3 mol L−1 (100.7–150.1) ppm. Thus the sensor can be used to detect fluoride ions in drinking water.
Because of the crucial roles of anions in biological systems and chemical processes, the design and synthesis of the molecular sensors that can detect the anions with specific selectivity are always of major interest.1–7 Among the range of anions, fluoride ion has attained significance because of its role in preventing dental decay8 and the treatment of osteoporosis.9 Thus, it is necessary to add fluoride to toothpaste and drinking water.10 However, fluoride is absorbed easily by the body and excreted slowly from the body.11 The presence of excess fluoride ions results in dental and skeletal fluorosis, bone diseases, mottling of teeth, lesions of the thyroid, liver and other organs.12–16 Therefore, as the World Health Organization limit is being followed in most of the nations, fluoride is considered beneficial in drinking water at levels of about 5.3 × 10−3 mol L−1 (100.7 ppm) but harmful once it exceeds 7.9 × 10−3 mol L−1 (150.1 ppm).17–19 Thus, the detection of fluoride ions has attracted considerable attention. Owing to its simplicity and exquisite sensitivity, fluorescent sensors are highly attractive for the selective detection of fluoride ions. Although a number of fluorescent sensors for fluoride ions have been developed in recent years.20–38 However, many of the reported sensors suffer from complicated synthesis procedures, turn-off fluorescence response, slow response or low sensitivity. Therefore, there is still a high demand for sensors for fluoride ions with good selectivity and high sensitivity.
Herein, based on our previous work,39–41 we report a chemosensor S based on 2-hydroxy-1-naphthalene formaldehyde Schiff base. The chemosensor S in DMSO solution could act as a colorimetric and turn-on fluorescent sensor for fluoride ions from aqueous medium. Upon the addition of water solution of fluoride ions, sensor S could immediately show remarkable color change and significant fluorescence enhancement. The detection limit for F− was determined to be 1.4 × 10−8 mol L−1 (2.66 × 10−4 ppm).
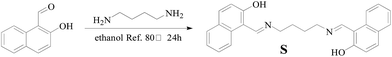
The synthetic route for sensor S is shown in Scheme 1. 2-Hydroxy-1-naphthalene formaldehyde Schiff base was obtained by refluxing 2-hydroxy-1-naphthalene formaldehyde (6 mmol, 1.033 g) with 1-4-butyl diamine (3 mmol, 0.264 g) in ethanol (50 mL) at 80 °C for 24 h. The obtained residue was recrystallized by alcohol to yield the product, S, as yellow solid (1.023 g, 86%) (Fig. S1†). 1H-NMR (d6-DMSO, 400 MHz) δ 14.17 (s, 2H, –O–H), 9.13 (s, 2H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.07 (d, 2H, J = 4 Hz, –ArH), 7.72 (d, 2H, J = 4 Hz, –ArH), 7.62 (d, 2H, J = 8 Hz, –ArH), 7.41 (t, 2H, J = 8 Hz, –ArH), 7.18 (t, 2H, J = 6 Hz, –ArH), 6.72 (d, 2H, J = 8 Hz, –ArH), 3.73 (s, 4H, CH
N), 8.07 (d, 2H, J = 4 Hz, –ArH), 7.72 (d, 2H, J = 4 Hz, –ArH), 7.62 (d, 2H, J = 8 Hz, –ArH), 7.41 (t, 2H, J = 8 Hz, –ArH), 7.18 (t, 2H, J = 6 Hz, –ArH), 6.72 (d, 2H, J = 8 Hz, –ArH), 3.73 (s, 4H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–CH2), 1.87 (s, 4H, –CH2). IR (Fig. S2†) (KBr, cm−1) ν: 3426 (–OH), 1634 (C
N–CH2), 1.87 (s, 4H, –CH2). IR (Fig. S2†) (KBr, cm−1) ν: 3426 (–OH), 1634 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); MS-ESI (Fig. S3†) calcd for C26H24N2O2 [L + H]+: 396.4810; found: 397.1322.
N); MS-ESI (Fig. S3†) calcd for C26H24N2O2 [L + H]+: 396.4810; found: 397.1322.
As shown in Fig. S4,† sensor S in DMSO was non-fluorescent in the absence of fluoride ions. Upon addition of 50 equiv. of fluoride ions in aqueous solution, a dramatic fluorescence emission enhancement (30-fold) was observed with a maximum at 467 nm (Fig. 1). Fluorescence of S (measured 0.5 mL 2.0 × 10−4 mol L−1 diluted to 2.0 × 10−5 mol L−1) in DMSO increased gradually when titrated with F− (measured 0.5 mL 0.1mol L−1 diluted to 0.01 mol L−1), and saturated at about 50 equiv. by exhibiting a 30-fold increase (Fig. 2). However, the addition of other anions couldn't induce similar response. There was a good linear correlation between the fluorescence intensity and the concentration of fluoride ions in the range of 0–50 equiv. (Fig. S5†). The linear equation was expressed as Y = 111.7847x − 3022.424 (R = 0.9964, where Y denotes the fluorescence intensity and x denotes the ratio of fluoride ion with S: n(F−)/n(S)). The detection limit was determined to be 1.4 × 10−8 M (2.66 × 10−4 ppm).42
In order to evaluate the selectivity of the sensor for fluoride ions, we investigated the fluorescence behavior of sensor S with other typical anions. The results presented in Fig. 3 show that other anions (Cl−, Br−, I−, AcO−, H2PO4−, HSO4−, ClO4−, using their the tetrabutylammonium (TBA) salts as sources; CN− and SCN−, using their sodium salts as sources.) in aqueous solution had no effect on the fluorescence behavior of sensor S, even at that high concentrations (50 equiv. of sensor S). Therefore, the sensor S displayed excellent selectivity for fluoride ions in aqueous solution over other competing anions.
Sensor S (2.0 × 10−5 mol L−1) in DMSO is colorless with an absorption maximum at 402 nm (Fig. S6†). Upon the addition of 50 equiv. of F− (0.01 mol L−1) in aqueous solution, an apparent red-shift near 20 nm (from 402 to 423 nm) instantly appeared. With increasing addition amounts of fluoride ions, the absorption band at 402 nm progressively decreased and a new band centered at 423 nm gradually formed with a clear isosbestic point at 340 nm. Meanwhile, the absorption spectra of sensor S at 428 nm underwent two different phases. At first phase, with increasing addition of fluoride ions, as shown from Fig. 4, an isosbestic point appeared at about 428 nm (signed by purple arrow). However, with continuous addition of fluoride ions, the absorption spectra of sensor S no longer cross the isosbestic point at 428 nm. Which indicated that sensor S interact with F− by hydrogen bonding at first phase, and deprotonation during addition of fluoride ions (second phase).
 | ||
| Fig. 4 UV/vis absorption spectra of sensor S (2.0 × 10−5 mol L−1) in DMSO containing different amounts of F− (0–50 equiv.) in aqueous solution. | ||
To demonstrate the selectivity of sensor S as a colorimetric sensor, similar experiments were carried out on sensor S with other typical anions (Cl−, Br−, I−, AcO−, H2PO4−, HSO4−, ClO4−, CN− and SCN−) by observing the absorption spectral change. As shown in Fig. S7,† only fluoride ions could induce the sensor to form a deep yellow color with a red-shift near 20 nm, whereas other typical anions had no influence on the absorption spectra. The above results proved that sensor S in DMSO could be used as a highly selective colorimetric sensor for fluoride ions in aqueous solution.
As known, chemosensors always have a problem of long response time. For example, reaction-based43 F− sensor or supramolecular gel-based F− sensor often need several minutes to response F−.44,45 In our case, the binding process of F− to S was found to be very fast (Fig. S8†). After adding the aqueous solution of fluoride ions, the fluorescence emission intensity of S increased at 467 nm and reached the plateau region less than 1 s, and remained better stable. These results indicated that the sensor S in DMSO could instantly detect F−.
The reversibility in the response of S has been demonstrated during its four cycles of titrations carried out with F− (blue) followed by HClO4 (black) in a sequence as shown in Fig. 5a. Further, after addition of F− the solution of sensor S exhibits a remarkable fluorescence change and shows by showing ON behaviour. Titration of this fluorescent complex with HClO4 results in quenching the fluorescence intensity and hence acts as OFF switch. The repeated demonstration of ON/OFF behaviour of the system by fluorescence as well as the visual color change clearly suggests that S in DMSO is reversible.
Since the fluorescence emission of sensor S could be accurately controlled by alternative addition of F− and H+ ions, the fluorescence emission of S can be defined as the logic gate output, which provides an entry for developing a molecular logic gate using H+ and F− as two inputs. For inputs, the addition and the absence of F− and H+ can be defined as 1 and 0, respectively. For output, we define the normalized fluorescence intensity at 420 nm of S enhance as 1 and not enhance as 0. Based on the above definitions, the fluorescence enhancing of S is observed only in the addition of F−, so that the output is read as “1”. Under other circumstances, the fluorescence of S is not enhanced leading to the output being read as “0”. The respective symbolic representation of the INHIBIT (INH)46 function and the corresponding truth table are illustrated in Fig. 5c. Thus S can act as INH logic gate.
To investigate the practical application of sensor S, test strips were prepared by immersing filter papers into a DMSO solution of S (0.1 M) and then drying in air. The test strips containing S were utilized to sense F−. As shown in Fig. S9,† when F− was added to the test kits, the obvious fluorescence change was observed under the 365 nm UV lamp. However, potentially competitive ions exerted no influence on the detection of F− by the test strips. Therefore, the test strips could conveniently detect F− in aqueous solution.
The further anion-S recognition mechanism was observed from 1H NMR titration experiments (Fig. 6) in DMSO-d6. Before the addition of anions, the 1H NMR chemical shifts of the O–H and Ar–Ha (Fig. 6 inset) protons on S were at δ 14.16 and 8.06 ppm, respectively. After adding 1 equiv. of fluoride ions, the resonances for O–H protons disappeared, and a weak broad signal appeared at δ 14.28 ppm, which indicated the formation of O–H⋯F hydrogen bonds. With continuous addition of fluoride ions, this signal disappeared, which suggested that the O–H groups perhaps underwent a deprotonation process. Simultaneously, a new weak broad signal appeared at δ 16.09 ppm, which indicated the formation of HF2−.47 Meanwhile, the Ar–Ha proton signal at δ 8.06 ppm showed a downfield-shift, which indicated the formation of C–Ha⋯N intramolecular hydrogen bonds, which led to a significant fluorescence enhancement and color change (Fig. 7).
Conclusions
In summary, we have developed a colorimetric and turn-on fluorescent sensor based on 2-hydroxy-1-naphthalene formaldehyde bis-Schiff base. The sensor S in DMSO displayed high sensitivity and good selectivity for the detection of fluoride ions in aqueous solution. Test strips based on S were prepared, which could conveniently detect F− in aqueous solution. The detection limit was 1.4 × 10−8 M (2.66 × 10−4 ppm), which is far lower than the WHO guideline of drinking water at levels of about (5.3–7.9) × 10−3 M [(100.7–150.1) ppm]. Thus the sensor can be used to detect fluoride ions in drinking water.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (no. 21064006; 21161018; 21262032), the Natural Science Foundation of Gansu Province (1308RJZA221) and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (IRT1177).Notes and references
- R. M. Duke, E. B. Veale, F. M. Pfeffer, P. E. Kruger and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39, 3936–3953 RSC.
- X. G. Li, D. Zhang and J. Li, Spectrochim. Acta, Part A, 2014, 127, 1–9 CrossRef CAS PubMed.
- S. S. Khan and M. Riaz, Talanta, 2014, 122, 209–213 CrossRef PubMed.
- K. Jakusová, J. Donovalová, M. Cigáň, M. Gáplovský, V. Garaj and A. Gáplovský, Spectrochim. Acta, Part A, 2014, 123, 421–429 CrossRef PubMed.
- B. B. Hu, P. Lu and Y. G. Wang, Sens. Actuators, B, 2014, 195, 320–323 CrossRef CAS PubMed.
- L. Wang, W. Li, J. Lu, J.-P. Zhang and H. Wang, Tetrahedron, 2014, 70, 3172–3177 CrossRef CAS PubMed.
- V. Suryanti, M. Bhadbhade, H. M. Chawla, E. Howe, P. Thordarson, D. S. C. Black and N. Kumar, Spectrochim. Acta, Part A, 2014, 121, 662–669 CrossRef CAS PubMed.
- J. D. B. Featherstone, Community Dent. Oral Epidemiol., 1999, 27, 31–40 CrossRef CAS PubMed.
- M. Kleerekoper, Endocrinol. Metab. Clin. North Am., 1998, 27, 441–452 CAS.
- J. S. Chen, P. W. Zhou, G. Y. Li, T. S. Chu and G. Z. He, J. Phys. Chem. B, 2013, 117, 5212–5221 CrossRef CAS PubMed.
- N. M. Mattiwala, R. Kamal and S. K. Sahoo, Res. Chem. Intermed., 2013, 1–10 Search PubMed.
- P. P. Singh, M. K. Barjatiya, S. Dhing, R. Bhatnagar, S. Kothari and V. Dhar, Urol. Res., 2001, 29, 238–244 CrossRef CAS.
- H. Matsui, M. Morimoto, K. Horimoto and Y. Nishimura, Toxicol. In Vitro, 2007, 21, 1113–1120 CrossRef CAS PubMed.
- O. Barbier, L. Arreola-Mendoza and L. M. Del Razo, Chem.-Biol. Interact., 2010, 188, 319–333 CrossRef CAS PubMed.
- C. D. Anuradha, S. Kanno and S. Hirano, Arch. Toxicol., 2000, 74, 226–230 CrossRef CAS.
- J. A. Camargo, Chemosphere, 2003, 50, 251–264 CrossRef.
- WHO (World Health Organization), Guidelines for Drinking Water Quality, World Health Organization, Geneva, 1985, vol. 3, pp. 1–2 Search PubMed.
- WHO (World Health Organization), Guideline for Drinking Water Quality Health criteria and other supporting information, World Health Organization, Geneva, 2nd edn, 1997, vol. 2 Search PubMed.
- NHMRC, Australian Drinking Water Guidelines, National Health and Medical Research Council, 2004, http://www.nhmrc.gov.au/publications/synopses/_files/adwg_11_06_fact_sheets.pdf Search PubMed.
- P. G. Sutariya, N. R. Modi, A. Pandya, B. K. Joshi, K. V. Joshia and S. K. Menon, Analyst, 2012, 137, 5491–5494 RSC.
- S. Velmathi, V. Reena, S. Suganya and S. Anandan, J. Fluoresc., 2012, 22, 155–162 CrossRef CAS PubMed.
- D. Sharma, S. K. Sahoo, R. K. Bera and R. Kamal, J. Fluoresc., 2013, 23, 387–392 CrossRef CAS PubMed.
- L. Zang, D. Wei, S. Wang and S. Jiang, Tetrahedron, 2012, 68, 636–641 CrossRef CAS PubMed.
- C. Wang, G. Li and Q. Zhang, Tetrahedron Lett., 2013, 54, 2633–2636 CrossRef CAS PubMed.
- S. Sharma, M. S. Hundal and G. Hundal, Tetrahedron Lett., 2013, 54, 2423–2427 CrossRef CAS PubMed.
- L. Y. Zhao, G. K. Wang, J. H. Chen, L. M. Zhang, B. Liu, J. F. Zhang, Q. H. Zhao and Y. Zhou, J. Fluorine Chem., 2014, 158, 53–59 CrossRef CAS PubMed.
- P. Hou, S. Chen and X. Song, Luminescence, 2014, 29, 423–426 CrossRef CAS PubMed.
- J. Nieboer, R. Haiges, W. Hillary, X. Yu, T. Richardet, H. P. A. Mercier and M. Gerken, Inorg. Chem., 2012, 51, 6350–6359 CrossRef CAS PubMed.
- Y. Hu, Z. Zhao, X. Bai, X. Yuan, X. Zhang and T. Masuda, RSC Adv., 2014, 4, 55179–55186 RSC.
- B. Zou, H. Liu, J. Mack, S. Wang, J. Tian, H. Lu, Z. Li and Z. Shen, RSC Adv., 2014, 4, 53864–53869 RSC.
- B. Deka and R. J. Sarma, Sens. Actuators, B, 2014, 147, 321–325 CrossRef PubMed.
- A. P. De Silva, I. M. Dixon, H. Q. N. Gunaratne, T. Gunnlaugsson, P. R. S. Maxwell and T. E. Rice, J. Am. Chem. Soc., 1999, 121, 1393–1394 CrossRef CAS.
- H. Zhao and F. P. Gabbai, Org. Lett., 2011, 13, 1444–1446 CrossRef CAS PubMed.
- G. J. Park, H. Y. Jo, K. Y. Ryu and C. Kim, RSC Adv., 2014, 4, 63882–63890 RSC.
- V. Lozano, R. Hernández, A. Ardá, J. Jiménez-Barbero, C. Mijangos and M.-J. Pérez-Pérez, J. Mater. Chem., 2011, 21, 8862 RSC.
- S. Lee, S. Oh, J. Lee, Y. Malpani, Y. S. Jung, B. Kang, J. Y. Lee, K. Ozasa, T. Isoshima, S. Y. Lee, M. Hara, D. Hashizume and J. M. Kim, Langmuir, 2013, 29, 5869–5877 CrossRef CAS PubMed.
- M. Park, D. Jang, S. Young Kim and J.-I. Hong, New J. Chem., 2012, 36, 1145 RSC.
- Y. M. Zhang, Q. Lin, T. B. Wei, D. D. Wang, H. Yao and Y. L. Wang, Sens. Actuators, B, 2009, 137, 447–455 CrossRef CAS PubMed.
- Y. M. Zhang, Q. Lin, T. B. Wei, X. P. Qin and Y. Li, Chem. Commun., 2009, 6074–6076 RSC.
- Q. Li, Y. Guo, J. Xu and S. Shao, J. Photochem. Photobiol., B, 2011, 103, 140–144 CrossRef CAS PubMed.
- J. Liu, Y.-Q. Xie, Q. Lin, B.-B. Shi, P. Zhang, Y.-M. Zhang and T.-B. Wei, Sens. Actuators, B, 2013, 186, 657–665 CrossRef CAS PubMed.
- Y. Peng, Y. M. Dong, M. Dong and Y. W. Wang, J. Org. Chem., 2012, 77, 9072–9080 CrossRef CAS PubMed.
- T. Nishimura, S. Y. Xu, Y. B. Jiang, J. S. Fossey, K. Sakurai, S. D. Bull and T. D. James, Chem. Commun., 2013, 49, 478–480 RSC.
- P. Rajamalli and E. Prasad, Org. Lett., 2011, 13, 3714–3717 CrossRef CAS PubMed.
- Q. Lin, X. Zhu, Y. P. Fu, Y. M. Zhang, R. Fang, L. Z. Yang and T. B. Wei, Soft Matter, 2014, 10, 5715–5723 RSC.
- M. Kumar, R. Kumar and V. Bhalla, Org. Lett., 2011, 13, 366–369 CrossRef CAS PubMed.
- S. O. Kang, D. Powell, V. W. Day and K. Bowman-James, Angew. Chem., Int. Ed., 2006, 45, 1921–1925 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, synthesis of P, and other materials. See DOI: 10.1039/c4ra09624j |
| This journal is © The Royal Society of Chemistry 2015 |