Nickel ferrite nanoparticles: an efficient and reusable nanocatalyst for a neat, one-pot and four-component synthesis of pyrroles
Abstract
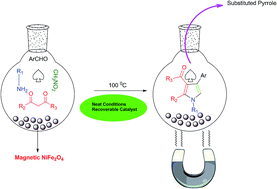
In this study nickel ferrite magnetic nanoparticles were applied as an efficient and reusable catalyst in the four-component synthesis of substituted pyrroles under neat conditions. The reaction was conducted using various amounts of catalyst at different temperatures and finally, application of 5 mol% of catalyst at 100 °C was determined as the optimum reaction condition. Results showed that nickel ferrite nanoparticles could catalyze the reaction at relatively short times (3–4 h) with high to excellent yields (70–96%). The catalyst could be recovered easily using an external magnetic field and reused nine times without any significant activity lost.

 Please wait while we load your content...
Please wait while we load your content...