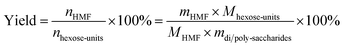Glucose dehydration to 5-hydroxymethylfurfural in ionic liquid over Cr3+-modified ion exchange resin
Hui Liu*,
Hongwei Wang,
Yuan Li,
Wei Yang,
Changhua Song,
Huaming Li*,
Wenshuai Zhu and
Wei Jiang
School of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang, 212013, P. R. China. E-mail: lihm@ujs.edu.cn; lh7544@ujs.edu.cn; Fax: +86-511-88791708; Tel: +86-511-88791800
First published on 10th December 2014
Abstract
In this work, D001-cc cation-exchange resin was modified by CrCl3 using an ion exchange method to obtain a Cr3+-D001-cc catalyst. FT-IR, UV-vis, SEM, EDX, ICP-OES and Py-IR were used to characterize the catalyst. Subsequently, the effects of the modified metal ion and different cation-exchange resin on the direct conversion of glucose to 5-hydroxymethylfurfural in [Bmim]Cl were examined. The results showed that Cr3+-D001-cc resin exhibited excellent catalytic performance. Moreover, with Cr3+-D001-cc resin as a catalyst, the influence of the reaction conditions on the dehydration of glucose was investigated in detail. 61.3% yield of 5-hydroxymethylfurfural was achieved in the [Bmim]Cl ionic liquid at 110 °C for 30 min with 0.1 g catalyst. Finally, a recycling experiment was performed. The result indicated that the HMF yield in the Cr3+-D001-cc resin and [Bmim]Cl system significantly decreased after six recycles.
1. Introduction
Nowadays, the demand for energy is globally increasing.1 However, fossil-fuels, as a major supply of energy, cannot meet the ever-increasing need because of the decreasing fossil-fuel reserves and the environmental pollution brought about by the utilization of fossil-fuel.2 Therefore, biomass, as an abundant, renewable, non-polluting and widespread novel energy resource, has attracted considerable attention in recent years.3–6 5-Hydroxymethylfurfural (HMF) is just an important intermediate in the utilization of biomass because it is a significant platform compound widely applied in the fine chemical field.7,8Both fructose and glucose are the normal monosaccharides used to prepare HMF, but compared with glucose, fructose can be converted more easily to HMF.9,10 However, the resource of fructose is not abundant in nature, which leads to high cost during the preparation of HMF. Therefore, glucose is more suitable than fructose for the mass industrial production of HMF. In recent years, the dehydration of glucose to prepare HMF has been investigated by many researchers.11–13 Despite these efforts, a high yield of HMF from glucose is difficult to achieve. Therefore, the search for catalytic systems is of great importance for the dehydration of glucose to HMF.
Solvent plays a key role in the catalytic system for the dehydration of glucose to HMF. At present, many solvents, including water, organic compounds, biphasic solvents and ionic liquids, are applied to the reaction.8,12,14,15 Among all these solvents, ionic liquids are promising solvents because of their unusual properties, such as high thermal and chemical stability, negligible vapor pressure, non flammability, and adjustable solvent power for organic substances.16,17
Another significant factor for the dehydration of glucose is the implementation of catalysts. Various catalysts have been used to catalyze the dehydration of glucose to HMF.18,19 From the results of these studies, metal ions, especially Cr3+ and Cr2+, exhibit excellent catalytic performance. In 2007, chromium dichloride (CrCl2) and chromium trichloride (CrCl3) were first used to catalyze the conversion of glucose to HMF in the presence of 1-ethyl-3-methylimidazolium chloride([Emim]Cl).20 The HMF yields of 70% and 45%, respectively, were obtained at 100 °C in 3 h. Subsequently, catalysts with chromium as the catalytic core were designed to catalyze the conversion of glucose to HMF. Hu et al. and Yong et al. used chromium(III) chloride (CrCl3·6H2O) in tetraethylammonium chloride (TEAC) ionic liquid and N-heterocyclic carbene/CrCl2(CrCl3) to catalyze the dehydration of glucose to HMF, respectively. The results showed that chromium ions had excellent catalytic performance for the dehydration of glucose to HMF.21,22 Moreover, Zhang et al. studied the dehydration of glucose and fructose catalyzed by CrII and CrIII chlorides by a combined experiment and computational study. The results indicated that the ability of chromium to selectively dehydrate glucose to HMF in [Emim]Cl did not depend on the oxidation state of chromium.23 Subsequently, Jia et al. reported that adjusting the solvent system was the most effective way of changing the product distribution with CrCl3·6H2O as a catalyst for the conversion of glucose to HMF. A controlled amount of water in the non-aqueous system was favorable for driving the thermodynamic equilibrium for a high HMF yield and desired product selectivity.24,25 However, the toxicity and pollution of chromium in the environment are well-known. Therefore, avoiding the drawbacks of chromium while achieving the appropriate catalytic activity is essential. As seen in the abovementioned studies, chromium cannot be separated from the reaction system because of its solubility in the solvent, leading to environmental damage. It is believed that utilizing an insoluble solid catalyst with Cr should be a better choice to decrease the damaging effects.
From our previous work,9 D001-cc cation-exchange resin has excellent catalytic performance for the dehydration of fructose to HMF and the HMF yield reaches 93.0% at 75 °C for 20 min. However, a HMF yield of 4.6% could be attained when glucose was used as the substrate. This might be because that D001-cc resin contains rich Brønsted acid sites, which favors the dehydration of fructose, but lacks Lewis acid sites. It was reported that in the dehydration process of glucose to HMF, glucose was isomerized first to fructose, which was then transformed to HMF by dehydration.26 During the process, the Lewis acid plays an important role. Different metal ions with Lewis acidity are exchanged easily into the resin by a simple ion-exchange method according to the characteristics of the cation-exchange resin. Moreover, homogeneous catalyst loaded on the resin can still maintain the catalytic activity,27 which suggests that introducing the metal ion into the cation-exchange resin can maintain their respective catalytic characteristics. At present, the metal-modified resin is applied to different organic reaction such as the Suzuki–Miyaura reaction and the esterification reaction.28–30 However, its catalytic performance for the dehydration of glucose to HMF has never been investigated.
Therefore, according to the above discussion, a series of cation-exchange resins modified by metal ion were prepared in this work. First, the effects of different modified metal ions and cation-exchange resins on the reaction were discussed to choose a suitable modified metal ion and support. Subsequently, the effects of the reaction temperature, solvent, catalyst dosages and different initial fructose loadings on the dehydration of glucose and the reusability of the resin/ionic liquid system were also investigated with the optimal resin as a catalyst in a favorable ionic liquid.
2. Experimental
2.1 Materials
HMF (99% purity) was purchased from Wutong Spice Co., Ltd. Fructose (B. R. grade), glucose (A. R. grade), hydrochloric acid (HCl, A. R. grade), ethanol (A. R. grade) and methanol (CH3OH, L. C. grade) were purchased from Sinopharm Chemical Reagent Co., Ltd. 1-Butyl-3-methylimidazolium chloride([Bmim]Cl) was purchased from Shanghai Cheng Jie Chemical Co., Ltd. All the chemicals were used without further purification. All Na-type cation-exchange resins were purchased from Nankai University Chemical Factory, including D001-cc, D072, D152, NKC-9, 001 × 1 and 001 × 7.2.2 Experimental methods
For fructose and glucose, the yield of HMF was determined using the following formula.
For disaccharides and polysaccharides, HMF yield was defined as follows:
3. Results and discussion
3.1 Characterization of catalyst
The content of Na and Cr in D001-cc resin before and after modification is listed in Table 1. It can be found in Table 1 that Na+ in D001-cc resin was not completely exchanged by H+ or by Cr3+ whether by acidification or modified by CrCl3 through the ion-exchanging method. However, a large amount of Na+ could be exchanged when D001-cc resin was immersed in the mixture solution of CrCl3 and HCl in comparison with the content of Na+ in H+-D001-cc resin and Cr3+-D001-cc resin.| Na (mg g−1) | Cr (mg g−1) | |
|---|---|---|
| D001-cc (unmodified) | 68 | — |
| H+-D001-cc (acidified resin) | 40 | — |
| Cr3+-D001-cc | 18 | 34 |
The UV-vis diffuse reflectance spectrum of samples is illustrated in Fig. 1. Two absorption peaks at about 236 nm and 270 nm were found in the spectrum of D001-cc resin and Cr3+-D001-cc, which were represented by the characteristic peak of benzene.28 Nonetheless, the absorption peaks in Cr3+-D001-cc were slightly blue-shifted and the intensity of the absorption peaks decreased relative to those in the D001-cc resin. The result indicates that the introduction of Cr3+ does not influence the framework of the resin but changes the distribution of the electron cloud in the conjugated system. Moreover, no new absorption peak at 400 nm was observed in Cr3+-D001-cc, showing that the coordination effect does not exist between Cr3+ and the benzene ring in the D001-cc resin.29 Compared with the resin D001-cc, two distinct absorption peaks at 435 nm and 625 nm in the spectrum of Cr3+-D001-cc were found, which were produced by the d–d transitions of Cr3+. This indicates that Cr3+ is in the form of octahedron coordination in the Cr3+-D001-cc.31 Therefore, when Cr3+ is introduced into D001-cc resin by the ion-exchange method, Cr3+ might interact with the oxygen atom of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the D001-cc resin, leading to the blue-shift of the absorption peak of benzene and a decrease in peak intensity.
O in the D001-cc resin, leading to the blue-shift of the absorption peak of benzene and a decrease in peak intensity.
The FT-IR spectra of D001-cc and Cr3+-D001-cc are shown in Fig. 2. Three characteristic bands at 1600 cm−1, 1500 cm−1 and 1450 cm−1 were observed in the spectra of D001-cc and Cr3+-D001-cc, which was the principal feature of aromatic compounds.32 This also indicates that the modification process by metal ions has no influence on the framework of D001-cc resin. This result is in agreement with that of UV-vis diffuse reflectance characterization. In addition, there were two bands at 1182 cm−1 and 1039 cm−1 in the FT-IR spectrum of D001-cc, which represented the asymmetric and symmetric stretching vibration of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.33,34 However, the band at 1182 cm−1 in the FT-IR spectrum of Cr3+-D001-cc was split into two absorption peaks at 1160 cm−1 and 1224 cm−1, respectively. Moreover, the intensity of the peak at 1039 cm−1 in Cr3+-D001-cc resin was reduced and a new band at 900 cm−1 was found after the modification of Cr3+. These results indicate that the modification of D001-cc by metal ions increase the asymmetric stretching vibration of the S
O, respectively.33,34 However, the band at 1182 cm−1 in the FT-IR spectrum of Cr3+-D001-cc was split into two absorption peaks at 1160 cm−1 and 1224 cm−1, respectively. Moreover, the intensity of the peak at 1039 cm−1 in Cr3+-D001-cc resin was reduced and a new band at 900 cm−1 was found after the modification of Cr3+. These results indicate that the modification of D001-cc by metal ions increase the asymmetric stretching vibration of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in resin.30 This might be because of the fact that S
O in resin.30 This might be because of the fact that S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O has an interaction with Cr3+, which leads to an increase in the nonequivalence of S
O has an interaction with Cr3+, which leads to an increase in the nonequivalence of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.
O.
The SEM images and EDS spectra of D001-cc and Cr3+-D001-cc resin are shown in Fig. 3. Fig. 3a1 and a2 exhibit SEM images of D001-cc resin; Fig. 3b1 and b2 show the SEM images of Cr3+-D001-cc resin. It can be observed from the images that the surface of the unmodified D001-cc resin was quite smooth and abundant pore channels were found on it. In contrast, the surface of D001-cc resin became rough but still had considerable pore canals after the modification with Cr3+. These results suggest that the introduction of Cr3+ has little influence on the structure of D001-cc resin. Fig. 3a3 and b3 present the EDS spectra of D001-cc and Cr3+-D001-cc resin, respectively. It can be noted that Cr3+ only exists in Cr3+-D001-cc resin, which indicates that Cr3+ can be actually loaded on the resin by a simple ion-exchange method. However, it is worth noting that Na+ can be found in Cr3+-D001-cc resin, indicating that Na+ is not completely exchanged with Cr3+ in the impregnating process. The result is in agreement with that shown in Table 1.
Fig. 4 shows the Py-IR spectra of D001-cc resin, H+-D001-cc resin and Cr3+-D001-cc resin. Generally, the vibration bands in the 1400–1650 cm−1 regions are applied to distinguish the Brønsted and Lewis acid sites. The bands at 1449 cm−1 and 1537 cm−1 belong to Lewis acidic sites and Brønsted acidic sites, respectively. The band at 1492 cm−1 corresponds to Brønsted and Lewis acidic sites (L + B).35–37 As shown in Fig. 4, only Lewis acidic sites were found in the D001-cc resin and Cr3+-D001-cc resin, but the band intensity for adsorbed pyridine on the Lewis acid sites increased and a slight blue-shift was observed in Cr3+-D001-cc resin compared with the D001-cc resin, indicating that the acidity of D001-cc resin increases after modification with Cr3+. In contrast, both Brønsted acid and Lewis acid were found in H+-D001-cc resin. As listed in Table 1, a part of Na+ in D001-cc resin is exchanged by the H+ of a hydrochloric acid solution in the acidified process, which results in the appearance of Brønsted acidic sites.
According to the above spectra, a diagrammatic sketch of the preparation of Cr3+-D001-cc resin is proposed, which is shown in Fig. 5. When D001-cc resin is immersed in the mixture solution of HCl and CrCl3, most of the Na+ (the exchangeable cation in resin) are exchanged with Cr3+ through an interaction between Cr3+ and SO3− (the fixed group in resin). The replaced Na+ enters the immersion solution.
3.2 Influence of the different ions on the catalytic activity
Table 2 shows the change of catalytic performance of D001-cc resin before and after modification. As shown in the table, unmodified D001-cc resin (entry 1) had little catalytic activity (HMF yield of 1.2%) for the dehydration of glucose at 110 °C for 30 min. After being acidified with HCl, Na+ was exchanged to H+ (entry 2), but the catalytic ability was not improved significantly under the same conditions (HMF yield of 9.7%), indicating that H+-D001-cc resin also had poor catalytic performance for the dehydration of glucose. Nevertheless, H+-D001-cc resin exhibited excellent performance for the conversion of fructose to HMF in a previous study and the HMF yield reached 93.0% at 75 °C for 20 min.9 Fig. 4 shows that H+-D001-cc resin contains rich Brønsted acidic sites, favoring the dehydration of fructose. However, when free Na+ in D001-cc resin was exchanged with Cr3+, the HMF yield increased to 61.3% (entry 3). This proves that the introduction of Cr3+ is advantageous in improving the catalytic performance of the D001-cc resin, which is due to the introduction of strong Lewis acidic sites after modification with Cr3+ (Fig. 4).| Entry | Ion | HMF yield/% |
|---|---|---|
| a Conditions: glucose 0.1 g, [Bmim]Cl 1.5 g, 110 °C, 30 min. | ||
| 1 | Na+ | 1.2 |
| 2 | H+ | 9.7 |
| 3 | Cr3+ | 61.3 |
| 4 | Cr2+ | 60.7 |
| 5 | Cu2+ | 15.3 |
| 6 | Mg2+ | 6.2 |
| 7 | Fe3+ | 14.4 |
| 8 | Al3+ | 20.2 |
| 9 | Mo5+ | 5.4 |
Next, the effects of different metal ions (including Cu2+, Mo5+, Fe3+, Mg2+, Al3+, Cr2+ and Cr3+) on the dehydration of glucose to HMF was investigated in [Bmim]Cl at 110 °C for 30 min and the result is also listed in Table 2. After the modification with different metal ions, D001-cc resins show higher catalytic activity than the acidic D001-cc resin, except for the Mg2+-D001-cc resin. This suggests that the introduction of a metal ion is in favor of the dehydration of glucose. A more than 60% yield of HMF was achieved in the presence of D001-cc modified with Cr2+ and Cr3+, whereas less than 20% yield of HMF was obtained with other ions modified D001-cc resin as a catalyst. This result is in agreement with that reported by Zhao.20 It was found that chromic salts can promote the isomerization of glucose to fructose, whereas other metal salts can only improve the mutarotation of α-glucopyranose anomer to β-glucopyranose anomer. Therefore, Cr2+-D001-cc resin and Cr3+-D001-cc resin exhibit better catalytic performance than the other resins. Considering the cost of CrCl2 and CrCl3, CrCl3 was selected as the modified metal halide to perform the following experiments.
3.3 Effect of different resin supports on the catalytic activity
The influence of six different resin supports modified with Cr3+ on the dehydration of glucose to HMF was checked at 110 °C for 30 min, including macroporous strong-acidic cation-exchange resins (D072, D001-cc and NKC-9), macroporous weak-acidic cation-exchange resin (D152), and gel strong-acidic cation-exchange resins (001 × 1 and 001 × 7). The result is displayed in Table 3. When macroporous strong-acidic cation-exchange resins (NKC-9, D072 and D001-cc) were applied as the catalyst support, the catalysts showed good catalytic performance and 48.8%, 50.3% and 61.3% yield of HMF were achieved, respectively. However, when the other types of resins were selected as the support, not more than 10% yield of HMF yields was reached. This suggests that macroporous strong-acidic cation-exchange resin exhibits better catalytic activity than the other types of resins due to the larger surface area, the stronger acidity and large number of macrospores. This result is consistent with our previous work.9 From Table 3, D001-cc resin was selected as the support for subsequent experiments.| Entry | Resin | HMF yield/% |
|---|---|---|
| a Conditions: glucose 0.1 g, [Bmim]Cl 1.5 g, catalyst 0.1 g, 110 °C, 30 min. | ||
| 1 | 001 × 1 | 4.8 |
| 2 | 001 × 7 | 5.0 |
| 3 | NKC-9 | 48.8 |
| 4 | D072 | 50.3 |
| 5 | D001-cc | 61.3 |
| 6 | D152 | 6.9 |
3.4 Effect of different temperature on the dehydration of glucose
The influences of different reaction temperatures on the dehydration of glucose were studied and the result is shown in Fig. 6. At low temperatures (80 °C and 90 °C), the HMF yield increased as the reaction proceeded. Moreover, the higher the temperature was, the faster was the reaction rate. When the reaction temperature was increased to 100 °C and 110 °C, the initial HMF yield showed obvious improvement, showing that a high temperature is beneficial for the dehydration of glucose to HMF. Unlike the reaction at low temperatures, the HMF yield at high temperatures subsequently decreased smoothly with increasing time. The time needed to reach the peak yield was lesser at 110 °C than at 100 °C. This might be because of the fact that high temperature accelerates not only the dehydration of glucose to HMF but also the side reactions during the process. HMF can be decomposed to levulinic acid and formic acid, and HMF also can polymerize with HMF, glucose or intermediate products,38 leading to a decrease in HMF yield. Therefore, 110 °C and 30 min were selected as the optimum condition for the dehydration of glucose to HMF. | ||
| Fig. 6 Influence of different temperatures on the conversion of glucose to HMF. Conditions: glucose 0.1 g, [Bmim]Cl 1.5 g, catalyst 0.1 g. | ||
3.5 Effect of catalyst dosage on the dehydration of glucose
The effects of the catalyst dosage on the dehydration of glucose to HMF are listed in Table 4. The yield of HMF first rapidly increased and then increased smoothly with increasing catalyst dosage. Only a yield of 0.7% HMF was obtained without the catalyst. When 0.05 g of Cr3+-D001-cc resin was added, a 52.9% yield of HMF was achieved. This result suggests that Cr3+-D001-cc resin can effectively catalyze the dehydration of glucose to HMF. Increasing the catalyst dosage from 0.05 g to 2.0 g resulted in an increase in the yield of HMF from 52.9% to 66.4%, but the rate of the increase lowered considerably. This means that the yield of HMF will become constant. In previous work, the addition of more than 0.1 g of D001-cc resin led to a decrease in the HMF yield for the dehydration of fructose.9 However, in this work, the yield of HMF constantly increased with increasing catalyst dosage. This suggests that Cr3+ can improve the stability of HMF by suppressing the side reactions to increase the HMF yield.20| Entry | The dosage of catalyst/g | HMF yield/% |
|---|---|---|
| a Conditions: glucose 0.1 g, [Bmim]Cl 1.5 g, 110 °C, 30 min. | ||
| 1 | 0 | 0.7 |
| 2 | 0.05 | 52.9 |
| 3 | 0.1 | 61.3 |
| 4 | 0.15 | 65.1 |
| 5 | 0.2 | 66.4 |
3.6 Influence of different initial dosage of glucose
The effect of different initial dosages of glucose on the reaction was investigated. According to Table 5, the HMF yield slowly decreased with increasing initial glucose dosage. When the initial dosage of glucose was 0.05 g, Cr3+-D001-cc showed a high catalytic activity, resulting in a 70.9% yield of HMF. This suggests that the excess catalyst dosage relative to glucose can supply more catalytic sites to accelerate the dehydration of glucose to HMF. This result is in agreement with that shown in Table 4. However, the catalyst cannot provide enough catalytic sites for the dehydration of glucose with an increase in the initial dosage of glucose, which leads to a decrease in the yield of HMF.| Entry | Initial dosage of glucose/g | HMF yield/% |
|---|---|---|
| a Conditions: [Bmim]Cl 1.5 g, Cr3+-D001-cc 0.1 g, 110 °C, 30 min. | ||
| 1 | 0.05 | 70.9 |
| 2 | 0.1 | 61.3 |
| 3 | 0.2 | 51.2 |
| 4 | 0.3 | 41.9 |
| 5 | 0.4 | 36.0 |
3.7 Conversion of di/poly-saccharides in catalytic system
To study the substrate scope of this catalytic system, five various sugars were investigated, including monosaccharide (glucose and fructose), disaccharide (maltose) and polysaccharide (cellulose and starch). As shown in Table 6, with the increasing degree of polymerization, the catalytic activity of this catalytic system gradually decreased. When glucose and fructose were selected as substrates, a 61.3% and 68.9% yield of HMF was achieved, respectively. For maltose, only a 22.1% yield was obtained. Little catalytic activity of Cr3+-D001-cc resin was observed with polysaccharides as a substrate. This indicates that this catalytic system cannot quickly catalyze complex hydrolysis during a short time. According to previous studies, the conversion of polysaccharide to HMF was quite complicated and H2O and high temperature were beneficial to the reaction.39,40 Therefore, the poor catalytic performance of Cr3+-D001-cc/[Bmim]Cl system for polysaccharide may result from the waterless environment and lower temperature employed in the experiment. Compared with H-type D001-cc,9 the HMF yield for the dehydration of fructose decreased from 93.0% to 68.9%. This might be because of the fact that the resin with the Lewis acidity does not benefit the dehydration of fructose or an increase in reaction temperature leads to some side reactions, which produces a soluble substance and insoluble polymers.41| Entry | Substrate | HMF yield/% |
|---|---|---|
| a Conditions: [Bmim]Cl 1.5 g, Cr3+-D001-cc 0.1 g, 110 °C, 30 min. | ||
| 1 | Glucose | 61.3 |
| 2 | Fructose | 68.9 |
| 3 | Maltose | 22.1 |
| 4 | Cellulose | 2.4 |
| 5 | Starch | 3 |
3.8 Catalytic system recyclability
As an important aspect of the Cr3+-D001-cc/[Bmim]Cl catalytic system, the recyclability was tested with a set of experiments. The dosage of Cr3+-D001-cc, [Bmim]Cl and glucose were 0.1 g, 1.5 g and 0.1 g, respectively. The reaction temperature and time were 110 °C and 30 min, respectively. The operating details were consistent with the previous work9 and the result is shown in Fig. 7. A relatively stable yield of HMF was observed in the first four experiments, but the catalytic activity of the Cr3+-D001-cc/[Bmim]Cl system showed an obvious decrease after the sixth run. This indicates that the Cr3+-D001-cc/[Bmim]Cl system can be reused, but the recycling time is limited. This may be caused by two aspects. On one hand, the maximum service temperature of D001-cc resin is between 100 °C and 120 °C. Long-term use at that temperature results in the damage of resin easily. The fragments, probably resulting from the Cr3+-D001-cc resin, were observed after the fifth and sixth recycling experiment. On the other hand, the polymer produced in the reaction may be adsorbed on the resin, thus poisoning the catalytic activity of the resin. | ||
| Fig. 7 Recycle of the catalytic system. Conditions: glucose 0.1 g, [Bmim]Cl 1.5 g, Cr3+-D001-cc 0.1 g, 110 °C, 30 min. | ||
It is interesting that the HMF yields in the two following recycling tests are even higher than that in the 1st run. This was possibly due to the residual HMF and unreacted feed stock in the previous runs, as reported by Wang et al.42 However, the difference in the HMF yields between the first and second tests in this work is larger than the result shown in the literature. It was reported that CrCl4− could be formed between free Cr3+ and [Bmim]Cl, which was advantageous to the conversion of glucose.20,43 Therefore, the reaction solution after the first test was analyzed by ICP-OES. The results showed that Cr existed in the solution, which indicates that the large increase in HMF yield in the second and third test may be the result of the formation of CrCl4−.
4. Conclusion
Cr3+ modified D001-cc catalyst could be easily prepared via an eco-friendly ion-exchanged method. The modified process had no influence on the structure of the resin. On the other hand, Cr3+ could be easily exchanged to the framework of D001-cc resin. Observed from the results of dehydration of glucose, chrome ion and macropore strong-acidic cation-exchange resin could improve the catalytic activity. A 61.3% yield of HMF was obtained with Cr3+-D001-cc resin as the catalyst and [Bmim]Cl as the solvent at 110 °C for 30 min. After six recycling tests, an obvious decrease in HMF yield was observed for the dehydration of glucose in the above system.Acknowledgements
The authors thank the financial support for this study by the National Natural Science Foundation of China (21106057 and 21376111) and Advanced Talents of Jiangsu University (10JDG143).References
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- J. A. Melero, J. Iglesias and A. Garcia, Energy Environ. Sci., 2012, 5, 7393–7420 CAS.
- I. Agirrezabal-Telleria, I. Gandarias and P. L. Arias, Catal. Today, 2014, 234, 42–58 CrossRef CAS PubMed.
- Y. Zhang, J. Pan, M. Gan, H. Ou, Y. Yan, W. Shi and L. Yu, RSC Adv., 2014, 4, 11664–11672 RSC.
- H. A. Kazem and M. T. Chaichan, Renewable Sustainable Energy Rev., 2012, 16, 6007–6012 CrossRef PubMed.
- M. Capik, A. O. Yilmaz and I. Cavusoglu, Renewable Energy, 2012, 46, 1–13 CrossRef PubMed.
- M. Walia, U. Sharma, V. K. Agnihotri and B. Singh, RSC Adv., 2014, 4, 14414–14418 RSC.
- V. V. Ordomsky, J. van der Schaaf, J. C. Schouten and T. A. Nijhuis, ChemSusChem, 2013, 6, 1697–1707 CrossRef CAS PubMed.
- Y. Li, H. Liu, C. H. Song, X. M. Gu, H. M. Li, W. S. Zhu, S. Yin and C. R. Han, Bioresour. Technol., 2013, 133, 347–353 CrossRef CAS PubMed.
- Z. H. Zhang, B. Liu and Z. Z. Zhao, Carbohydr. Polym., 2012, 88, 891–895 CrossRef CAS PubMed.
- J. H. He, Y. T. Zhang and E. Y.-X. Chen, ChemSusChem, 2013, 6, 61–64 CrossRef CAS PubMed.
- F. Liu, M. Audemar, K. D. Vigier, D. Cartigny, J. M. Clacens, M. F. C. Gomes, A. A. H. Padua, M. C. Gomes, F. De Campo and F. Jerome, Green Chem., 2013, 15, 3205–3213 RSC.
- Y. Shen, J. Sun, Y. Yi, B. Wang, F. Xu and R. Sun, J. Mol. Catal. A: Chem., 2014, 394, 114–120 CrossRef CAS PubMed.
- Q. H. Ren, Y. Z. Huang, H. Ma, F. Wang, J. Gao and J. Xu, Bioresources, 2013, 8, 1563–1572 Search PubMed.
- E. F. Dunn, D. J. Liu and E. Y.-X. Chen, Appl. Catal., A, 2013, 460, 1–7 CrossRef PubMed.
- H. Zhao and G. A. Baker, J. Chem. Technol. Biotechnol., 2013, 88, 3–12 CrossRef CAS.
- L. Wu, J. Song, B. Zhang, B. Zhou, H. Zhou, H. Fan, Y. Yang and B. Han, Green Chem., 2014, 16, 3935–3941 RSC.
- Y. Yang, C. Hu and M. M. Abu-Omar, J. Mol. Catal. A: Chem., 2013, 376, 98–102 CrossRef CAS PubMed.
- Z. Hu, B. Liu, Z. H. Zhang and L. Q. Chen, Ind. Crops Prod., 2013, 50, 264–269 CrossRef CAS PubMed.
- H. B. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS PubMed.
- G. Yong, Y. G. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2008, 47, 9345–9348 CrossRef CAS PubMed.
- L. Hu, Y. Sun and L. Lin, Ind. Eng. Chem. Res., 2012, 51, 1099–1104 CrossRef CAS.
- Y. M. Zhang, E. A. Pidkl and E. J. M. Hensen, Chem.–Eur. J., 2011, 17, 5281–5288 CrossRef CAS PubMed.
- S. Jia, K. Liu, Z. Xu, P. Yan, W. Xu, X. Liu and Z. C. Zhang, Catal. Today, 2014, 234, 83–90 CrossRef CAS PubMed.
- S. Jia, Z. Xu and Z. C. Zhang, Chem. Eng. J., 2014, 254, 333–339 CrossRef CAS PubMed.
- S. Dutta, S. De and B. Saha, Biomass Bioenergy, 2013, 55, 355–369 CrossRef CAS PubMed.
- P. Barbaro and F. Liguori, Chem. Rev., 2009, 109, 515–529 CrossRef CAS PubMed.
- M. M. Zhang, M. M. Jiang and C. H. Liang, Chin. J. Catal., 2013, 34, 2161–2166 CrossRef CAS.
- F. Zhang, X. Y. Jiang, J. J. Hong, H. Lou and X. M. Zheng, Chin. J. Catal., 2010, 31, 666–670 CAS.
- H. Z. Jiang and A. P. Liao, Appl. Chem. Ind., 2011, 40, 246–251 CAS.
- M. G. Song, B. B. Fang, C. Jin and R. F. Li, Petrochem. Technol., 2005, 34, 927–931 CAS.
- S. Subashchandrabose, C. Meganathan, Y. Erdogdu, H. Saleem, C. Jajkumar and P. Latha, J. Mol. Struct., 2013, 1042, 37–44 CrossRef CAS PubMed.
- H. Li, Q. Y. Zhang, X. F. Liu, F. Chang, Y. P. Zhang, W. Xue and S. Yang, Bioresour. Technol., 2013, 144, 21–27 CrossRef CAS PubMed.
- H. Naeimi and Z. S. Nazifi, J. Nanopart. Res., 2013, 15, 2026–2037 CrossRef PubMed.
- T. Zhang, J. Liu, D. X. Wang, Z. Zhao, Y. C. Wei, K. Cheng, G. Y. Jiang and A. J. Duan, Appl. Catal., B, 2014, 148, 520–531 CrossRef PubMed.
- M. H. Peyrovi, N. Parsafard and P. Peyrovi, Ind. Eng. Chem. Res., 2014, 53, 14253–14262 CrossRef CAS.
- H. Zhang, X. Liu, M. Lu, X. Hu, L. Lu, X. Tian and J. Ji, Bioresour. Technol., 2014, 169, 800–803 CrossRef CAS PubMed.
- Y. Roman-Leshkov, M. Moliner, J. A. Labinger and M. E. Davis, Angew. Chem., Int. Ed., 2010, 49, 8954–8957 CrossRef CAS PubMed.
- N. Shi, Q. Liu, L. L. Ma, T. J. Wang, Q. Zhang and Q. Zhang, RSC Adv., 2014, 4, 4978–4984 RSC.
- J. J. Wang, J. W. Ren, X. H. Liu, J. X. Xi, Q. N. Xia, Y. H. Zu, G. Z. Lu and Y. Q. Wang, Green Chem., 2012, 14, 2506–2512 RSC.
- Y. Roman-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef CAS PubMed.
- P. Wang, H. B. Yu, S. H. Zhan and S. Q. Wang, Bioresour. Technol., 2011, 102, 4179–4183 CrossRef CAS PubMed.
- C. H. Song, H. Liu, Y. Li, S. Ge, H. W. Wang, W. S. Zhu, Y. H. Chang, C. R. Han and H. M. Li, Chin. J. Chem., 2014, 32, 434–442 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |