Hansen solubility parameters as a useful tool in searching for solvents for soy proteins†
Abolfazl Aghanouri and
Gang Sun*
Agricultural and Environmental Chemistry, Division of Textiles and Clothing, University of California, Davis, CA 95616, USA. E-mail: gysun@ucdavis.edu; Fax: +1 530 752 7584
First published on 19th November 2014
Abstract
Plant proteins as sustainable sources of biomacromolecules could be utilized as materials if proper and processable solvents can be identified. In an effort to search for proper solvents for soy proteins, we found that Hansen solubility parameters could be used as a useful tool to predict solvent systems that are likely to dissolve significant amount of the plant proteins.
Proteins mostly consist of 20 different amino acids with different side-chains in a specific sequence and possess special conformational structures as biomacromolecular chains. Plant proteins are abundant resources of biomacromolecules which are widely available as by-products of cooking oil productions, such as soy protein1 and corn zein,2 and could be the substitutes of petroleum oil based synthetic polymers.3 There are structural similarities between synthetic polymers and natural proteins, both having long linear backbone chains with short side chains and consisting of smaller subunits or building blocks. However, major differences do exist mostly in the structural complexity of the proteins in comparison with the synthetic polymers, caused by the variety of the amino acids as monomeric units. Amino acids possessing different structural features including hydrophilic and hydrophobic, making the biopolymers having different intermolecular interactions, generate the structural complexity of the proteins, while simple repeating units in synthetic polymers structures constituting unique and simple interactions. Consequently, the aforementioned complexity of the proteins brings some difficulties to find an appropriate solvent to dissolve and process them. High concentration of chaotropic agent solutions such as 6–8 M urea or guanidine hydrochloride solutions can dissolve most of the proteins to certain levels.4 Since urea and guanidine hydrochloride are solid materials, and they are dissolved in water, in manufacturing process after solidification of the protein solutions considerable amount of chaotropic agents will be left over as solids, which is even more than the amount of proteins by mass. Therefore, the use of the chaotropic agents is practically impossible in processing soy proteins into materials; and alternative liquids and processable solvents should be explored.
Traditionally to find a proper solvent for a synthetic polymer in polymer science solubility theories5 are used. These theories are based on an assumption that a proper solvent should have same physiochemical properties with the solute. Hildebrand solubility parameter was established based on square root of cohesive energy density of the material known as a solubility parameter of that compound.6 A solvent with a same or very close Hildebrand solubility parameter, δ, of a solute potentially has this ability to dissolve the solute. Hansen further expanded this theory and dissociated cohesive energy density into three components, 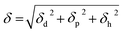 , where δd is related to intermolecular dispersion forces, δp is corresponded with intermolecular polar interactions and δh is referred to intermolecular hydrogen bonding.7 Hansen solubility parameters (HSPs) have been widely used in polymer science during last several decades. HSPs of solvents and polymers can be measured or predicted.7 It is popular to predict HSPs of a new polymer based on the chemical structures of repeating units in polymer and find either a single solvent or a solvent system which is matched with solubility parameters of the polymer.
, where δd is related to intermolecular dispersion forces, δp is corresponded with intermolecular polar interactions and δh is referred to intermolecular hydrogen bonding.7 Hansen solubility parameters (HSPs) have been widely used in polymer science during last several decades. HSPs of solvents and polymers can be measured or predicted.7 It is popular to predict HSPs of a new polymer based on the chemical structures of repeating units in polymer and find either a single solvent or a solvent system which is matched with solubility parameters of the polymer.
Solubility parameter distance, R, is another useful parameter to evaluate the strength of a solvent to dissolve a specific solute, and it is calculated based on differences of their partial solubility parameter components as R2 = 4(δd2 − δd1)2 + (δp2 − δp1)2 + (δh2 − δh1)2. HSPs were used to predict other behavior of the biomaterials such as gelation behavior,8 and several researches have been done on applications of HSPs in protein and biomaterials, but none of them produced any promising results.9 However, these research activities provided very useful clues in applying the HSP in searching proper solvents for soy proteins. In this paper we present a new approach to find proper liquid solvents for making processable plant protein solutions. Prediction of HSPs of the proteins has been quite difficult and inaccurate to perform due to their complex structures and different types of intermolecular interactions involved in the molecules. However, due to the fact of that urea solutions are universal solvents of the soy proteins,10 HSPs of the urea solutions were calculated as representatives of the protein's HSPs. Thus, the newly selected solvents and solvent systems will be calculated to match the HSPs of the urea solutions. For this purpose, soy proteins, glycinin11 and β-conglycinin,12 two purified major components,13 were selected as representative macromolecules. Organic solvents such as 2-chloroethanol, 2-butanol, 1,4-dioxane, dimethyl sulfoxide (DMSO), ethanol, 2-propanol, n-butanol, formamide, n-methylformamide, n-methylacetamide, triethanolamine, 2-pyrrolidone, propylene carbonate and glycerol carbonate were selected for making different solvent systems based on their different HSPs and high miscibility with water as the major solvent. This wide range of different organic solvents provides different intermolecular interactions such as, polar, nonpolar, protic, aprotic, and with different functional groups, such as halogens, alcohols, amines, amides, ethers, and sulfoxides. 100 different solvent systems were prepared by mixing the listed solvents in different compositions (volume%) and ratios. The solvent systems compositions are available in ESI.† Also, urea solutions in different urea molarities (1–6 M with 1 M intervals) were made to calculate and compare their HSP values with the organic solvent systems. The soy protein solubilities in all solvents were measured with 2% protein content in the solutions. The solutions were mixed, centrifuged and the remaining protein content was measured by Bradford reagent.14
Fig. 1 shows the HSPs of all tested solvent systems in a HSPs space diagram. The solvent systems with solubility higher than 40% for both purified soy proteins (glycinin and β-conglycinin) were depicted by red color, while solvents systems with poor solubility (less than 40%) with black color, primary solvents (i.e. pure non-aqueous solvents) with purple color, urea solutions with green color, and water with blue color, respectively.
It should be pointed out that the prediction and preparation of soy protein solvent systems by using the HPS method was possible since the good solvents do come very close to the targeted values of urea solutions, indicating the consistence with experimental results. But there are exceptions, and also it seems difficult to provide a smaller R to a 6 M urea solution by only using regular liquid organic solvents. One exception is water, which has a HSP close to the urea solutions. But pure water is not a perfect solvent for soy protein since it does not contain any hydrophobic element that can interfere with the hydrophobic area of the proteins. Also two other solvents (black color), though not good for dissolving the proteins, are in the same region. However, these exceptions can be all explained reasonably.
Fig. 2 demonstrates solubility distance, R, of the solvent systems and their solubility. Most of the solvents with R < 6 may have this potential to dissolve the proteins in a significant amount except sample numbers 113 and 114. Despite of the fact that these two solvent systems have R of 2.99 and 2.10, respectively, they demonstrated very weak solubility. These two solvent systems contain propylene carbonate and glycerol carbonate and water. Comparing the solvent compositions of the good solvent systems with these two solvent systems, it was found that all of others contain amine or amide groups in their chemical structures, including triethanolamine, 2-pyrrolidone, n-methylformamide and formamide, different from the solvent system numbers 113 and 114 without any nitrogen in their structures. In fact, for a good solvent system in addition to have proper HSP values, the solvent should have functional groups similar to urea or peptide bonds, which is the basic principle in selecting solvents and also the foundation for the development of solubility parameters. In another words, these solvents should be considered at the beginning of testing HSPs. Thus, we believe that the Hansen solubility parameters (HSP), are useful in identifying potential solvents for biopolymers.
To have a quantitative comparison, solubility values of the solvents for glycinin and β-conglycinin are listed in Table 1. The results indicate that the samples with smaller R values have higher capacity to dissolve proteins. In conclusion, we found that Hansen solubility parameters could predict solubility behavior of organic solvent systems for soy proteins, if the candidate solvents could meet the basic principle of structural similarity to the biopolymers. Thus, a solvent system with a small R to high concentration urea solutions can potentially dissolve the protein. It is an easy and quick method to search for potential solvents for proteins.
| Sample no. | Ra | Glycinin solubility (%) | β-Conglycinin solubility (%) |
|---|---|---|---|
| 108 | 2.58 | 87.38 ± 0.37 | 95.22 ± 0.27 |
| 111 | 3.34 | 80.80 ± 0.41 | 93.51 ± 0.18 |
| 109 | 3.90 | 68.81 ± 0.58 | 79.50 ± 0.38 |
| 112 | 4.86 | 51.78 ± 0.66 | 69.72 ± 0.30 |
| 102 | 5.13 | 68.91 ± 0.36 | 85.22 ± 0.33 |
| 110 | 5.60 | 69.51 ± 0.59 | 84.21 ± 0.21 |
| 58 | 5.92 | 43.57 ± 0.43 | 64.67 ± 0.43 |
| 99 | 6.41 | 49.01 ± 0.51 | 77.00 ± 0.37 |
| 103 | 6.31 | 53.18 ± 0.60 | 76.97 ± 0.52 |
| 6 M urea | 0.00 | 86.18 ± 0.23 | 94.99 ± 0.29 |
| Water | 5.25 | 27.02 ± 0.72 | 55.65 ± 0.49 |
Acknowledgements
A.A acknowledges the Jastro Shields research award.Notes and references
- (a) K. Tian, Z. Shao and X. Chen, Biomacromolecules, 2010, 11, 3638 CrossRef CAS PubMed; (b) F. Song, D. Tang, X. Wang and Y. Wang, Biomacromolecules, 2011, 12, 3369 CrossRef CAS PubMed; (c) A. J. Poole, J. S. Church and M. G. Huson, Biomacromolecules, 2009, 10, 1 CrossRef CAS PubMed; (d) J. W. Rhim, A. Gennadios, A. Handa, C. L. Weller and M. A. Hanna, J. Agric. Food Chem., 2000, 48, 4937 CrossRef CAS PubMed; (e) R. Kumar, V. Choudhary, S. Mishra, I. K. Varma and B. Mattiason, Ind. Crops Prod., 2002, 16, 155 CrossRef CAS; (f) A. Gennadios, A. H. Brandenburg, C. L. Weller and R. F. Testin, J. Am. Chem. Soc., 1981, 103, 6062 CrossRef.
- (a) Y. P. Neo, S. Ray, A. J. Easteal, M. G. Nikolaidis and S. Y. Quek, J. Food Eng., 2012, 109, 645 CrossRef CAS PubMed; (b) S. W. Selling, A. Biswas, A. Patel, D. J. Walls, C. Dunlap and Y. Wei, Macromol. Chem. Phys., 2007, 208, 1002 CrossRef; (c) T. Gillgren, S. A. Barker, P. S. Belton, D. M. R. Georget and M. Stading, Biomacromolecules, 2009, 10, 1135 CrossRef CAS PubMed.
- (a) S. H. Patel and M. Xanthos, Adv. Polym. Technol., 2001, 20, 22 CrossRef CAS; (b) S. A. Miller, A. E. Landis and T. L. Theis, Environ. Sci. Technol., 2007, 41, 5176 CrossRef CAS; (c) J. D. Hamilton, K. H. Reinert and M. B. Freeman, Environ. Sci. Technol., 1994, 28, 186 CrossRef PubMed; (d) A. M. T. Tabone, J. J. Cregg, E. J. Beckman and A. E. Landis, Environ. Sci. Technol., 2010, 44, 8264 CrossRef PubMed; (e) S. A. Miller, ACS Macro Lett., 2013, 2, 550 CrossRef CAS.
- (a) B. J. Bennion and V. Daggett, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 5142 CrossRef CAS PubMed; (b) X. Mo and J. Sun, J. Am. Oil Chem. Soc., 2001, 78, 867 CrossRef CAS PubMed.
- (a) J. Gmehling, T. F. Anderson and J. M. Prausnitz, Ind. Eng. Chem. Fundam., 1978, 17, 269 CrossRef CAS; (b) J. Gmehling, J. Li and M. A. Schiller, Ind. Eng. Chem. Res., 1993, 32, 178 CrossRef CAS; (c) C. Reichardt and T. Welton, Solvents and Solvent Effects in Organic Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2011 Search PubMed.
- (a) M. J. Kamlet, P. W. Carr, R. W. Taft and M. H. Abraham, J. Am. Chem. Soc., 1981, 103, 6062 CrossRef CAS; (b) M. C. Stumpe and H. Grubmüller, J. Am. Chem. Soc., 2007, 129, 16126 CrossRef CAS PubMed.
- C. Hansen, Hansen Solubility Parameters: A User's Handbook, CRC Press, Boca Raton, Fla, 2nd edn, 2007 Search PubMed.
- (a) M. Raynal and L. Bouteiller, Chem. Commun., 2011, 47, 8271–8273 RSC; (b) J. Gao, S. Wu and M. A. Rogers, J. Mater. Chem., 2012, 22, 12651 RSC.
- (a) C. M. Hansen, Ind. Eng. Chem. Prod. Res. Dev., 1969, 8, 2–11 CrossRef CAS; (b) C. Hansen, Hansen Solubility Parameters: A User's Handbook, CRC Press, Boca Raton, Fla, 2nd edn, 2007 Search PubMed.
- (a) M. C. Stumpe and H. Grubmuller, J. Am. Chem. Soc., 2007, 129, 16126 CrossRef CAS PubMed; (b) A. Wallqvist, D. G. Covell and D. Thirumalai, J. Am. Chem. Soc., 1998, 120, 427 CrossRef CAS.
- (a) N. Catsimpoolas, J. A. Kenney, E. W. Meyer and B. F. Szuhaj, J. Sci. Food Agric., 1971, 22, 448 CrossRef CAS; (b) K. Kitamura, Y. Toyokawa and K. Harada, Phytochemistry, 1980, 19, 1841 CrossRef CAS.
- (a) K. Shibasaki, J. Agric. Food Chem., 1978, 26, 692 CrossRef; (b) Food Proteins and Their Applications, ed. S. Damodaran and A. Paraf, Marcel Dekker, USA, 1997 Search PubMed.
- (a) V. V. Suchkov, I. A. Valeriy, Y. Grinberg and V. B. Tolstogusov, J. Agric. Food Chem., 1990, 38, 92 CrossRef CAS; (b) T. Nagano, M. Hirotsuka, H. Mori, K. Kohyama and K. Mishinari, J. Agric. Food Chem., 1992, 40, 941 CrossRef CAS; (c) S. Wu, P. A. Murphy, L. A. Johnson, M. A. Reuber and A. R. Fratzke, J. Agric. Food Chem., 2000, 48, 2702 CrossRef CAS PubMed.
- (a) M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS; (b) T. Zor and Z. Selinger, Anal. Biochem., 1996, 236, 302–308 CrossRef CAS PubMed; (c) J. E. Noble and M. J. A. Bailey, Methods Enzymol., 2009, 463, 73–95 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Solvent system composition details. See DOI: 10.1039/c4ra09115a |
| This journal is © The Royal Society of Chemistry 2015 |


