Open Access Article
Open Access ArticleSynthesis of new copper(I) based linear 1-D-coordination polymers with neutral imidazolinium-dithiocarboxylate ligands†
A.
Neuba
*a,
J.
Ortmeyer
a,
D. D.
Konieczna
b,
G.
Weigel
b,
U.
Flörke
a,
G.
Henkel
a and
R.
Wilhelm
*b
aUniversity of Paderborn, Department of Chemistry, Inorganic Chemistry Section, Warburgerstr. 100, 33098 Paderborn, Germany. E-mail: adam.neuba@uni-paderborn.de; Fax: +49 5251 603319; Tel: +49 5251 602165
bUniversity of Paderborn, Department of Chemistry, Organic Chemistry Section, Warburgerstr. 100, 33098 Paderborn, Germany. E-mail: rene.wilhelm@uni-paderborn.de; Fax: +49 5251 603245; Tel: +49 5251 605766
First published on 15th December 2014
Abstract
An imidazolinium-dithiocarboxylate betaine has been applied for the first time as a ligand in a coordination polymer with copper(I) halides. The resulting 1-D-coordination compounds show a linear double chain structure with trigonally coordinated copper(I). The potential of the material as a heterogeneous photocatalyst was explored.
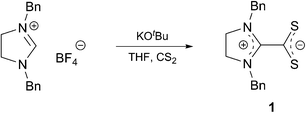
Imidazol(in)ium-dithiocarboxylate betaines (NHC·CS2) are formal adducts of a corresponding imidazol(in)ium carbene and CS2. The plane of the negatively charged CS2 group is almost perpendicular to the positively charged imidazolium ring. Compared to their CO2 and COS analogues,1 the CS2 betaines are known to be stable.1,2 After these betaines were described for the first time by Winberg and Coffmann,3 several groups focused their interests towards their chemical behaviour, for example, in (3 + 2) cycloadditions with electron deficient alkynes.4 Their application as ligands resulted in several reported metal complexes.5 The latter were investigated in a few catalytic reactions.6 Moreover, Delaude et al. have shown that stereoelectronic parameters of N-heterocyclic carbenes can be determined via XRD analysis of their CS2 adducts due to their outstanding ability to crystallize.7
Based on our interest in these betaines, we have recently shown that symmetric imidazolinium-dithiocarboxylates can be successfully applied as organocatalysts for the TMSCN addition to aldehydes8 and enantiopure analogues as organocatalysts for an asymmetric Staudinger reaction.9 Furthermore, we were able to synthesize a novel class of ionic liquids via methylation of the corresponding betaines. Spectroscopic analysis showed that the resulting red cations have different absorption maxima depending on the solvent and the lipophilicity of the corresponding counter anions.10 More recently, we reported the application of NHC·CS2 as colorimetric chemosensors for the detection of Hg2+ and Ag+.11 As a contribution to a better understanding of the properties of these betaines, we present here their application as neutral ligands for the preparation of copper(I) based 1-D-coordination polymers.
Coordination polymers represent an increasingly important class of new functional materials, and the number of publications has increased exponentially since the mid 1990's.12 This is due to their high potential in magnetic materials,13 catalysis,14 gas sorption,15 sensing16 and optoelectronics.17 In the present study, the potential of the new material was investigated as a heterogeneous photocatalyst.
Our approach starts with the synthesis10 (Scheme 1) of the betaine Bz2ImCS2 (1) and the determination of its hitherto unknown molecular structure (Fig. 1) for a comparative structural discussion with copper complexes derived thereof.
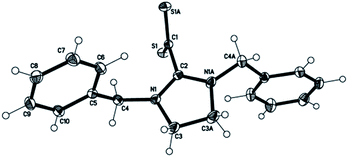 | ||
| Fig. 1 Molecular structure of 1 in the crystalline state.† | ||
Molecule 1 shows equal C–S and C–N bond lengths of 1.669(1) and 1.321(1) Å, respectively, and S–C–S as well as N–C–N valence angles of 123.5(1)° and 112.8(1)° (Table 1). The CS2/CN2 planes deviate significantly from an orthogonal arrangement exhibiting a dihedral angle of 79.3°. These parameters are comparable to structural data of other crystallised betaines with aromatic and aliphatic N substituents with dihedral angles ranging from 76.5° to 87.9°.4c,4e,7,18
| Atoms | Bond length | Atoms | Bond angle |
|---|---|---|---|
| 1 | |||
| S1–C1 | 1.669(1) | S1–C1–S1A | 130.68(10) |
| C1–C2 | 1.493(2) | S1–C1–C2 | 114.66(5) |
| C2–N1 | 1.321(1) | C1–C2–N1 | 124.01(8) |
| N1–C3 | 1.470(2) | N1–C2–N1A | 111.97(15) |
| C3–C3A | 1.530(3) | C2–N1–C3 | 110.98(11) |
| N1–C3–C3A | 102.92(7) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| 2 | |||
| Cu1–S1 | 2.244(1) | S1–Cu1–S2A | 107.41(3) |
| Cu1–S2A | 2.243(1) | S1–Cu1–Cl1 | 126.89(3) |
| Cu1–Cl1 | 2.194(1) | S2A–Cu1–Cl1 | 125.66(3) |
| C4–S1 | 1.682(3) | C4–S1–Cu1 | 112.77(10) |
| C4–S2 | 1.678(3) | C4–S2–Cu1A | 113.52(11) |
| C1–C4 | 1.482(4) | S1–C4–S2 | 124.18(18) |
| C1–N1 | 1.317(4) | S1–C4–C1 | 117.9(2) |
| C1–N2 | 1.322(3) | S2–C4–C1 | 117.9(2) |
| C4–C1–N1 | 123.8(3) | ||
| C4–C1–N2 | 124.0(3) | ||
| N1–C1–N2 | 112.2(2) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| 3 | |||
| Cu1–S1 | 2.244(5) | S1–Cu1–S2A | 110.53(2) |
| Cu1–S2A | 2.245(4) | S1–Cu1–Br1 | 125.39(1) |
| Cu1–Br1 | 2.323(3) | S2A–Cu1–Br1 | 124.02(2) |
| C4–S1 | 1.680(2) | C4–S1–Cu1 | 114.49(5) |
| C4–S2 | 1.683(2) | C4–S2–Cu1A | 115.11(5) |
| C1–C4 | 1.487(2) | S1–C4–S2 | 123.55(9) |
| C1–N1 | 1.319(2) | S1–C4–C1 | 118.50(11) |
| C1–N2 | 1.316(2) | S2–C4–C1 | 117.95(11) |
| C4–C1–N1 | 123.45(13) | ||
| C4–C1–N2 | 123.78(13) | ||
| N1–C1–N2 | 112.78(13) | ||
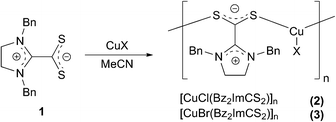
Reaction of 1 with CuX (X = Cl, Br) in acetonitrile (Scheme 2) leads to deep coloured (red, X = Cl; brown, X = Br) suspensions, from which after filtration and subsequent treatment with diethyl ether results in brown (X = Cl) or orange (X = Br) crystals that separate in the course of a few days. Interestingly, X-ray diffraction studies revealed that compounds 2 and 3 are coordination polymers of composition [CuX(Bz2ImCS2)]n, in which single 1D-chains are formed by alternately arranged {CuX} units and betaine ligands (Fig. 2).
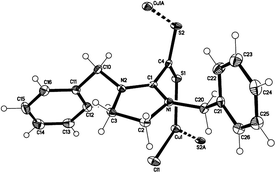 | ||
| Fig. 2 Section of the structure of 2 in the crystalline state.† | ||
Both coordination polymers crystallise isostructurally in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with Cu(I) atoms adopting trigonal-planar coordination geometries within their S2X donor sets (mean valence angle 119.99° within 2 and 119.98° within 3; see Table 1). The Cu–S bonds are equal in length and average to 2.244 Å in both 2 and 3. Pairs of polymeric chains are linked via intermolecular Cbenzyl–H⋯S interactions with H⋯S distances of 2.84 (2) and 2.79 Å (3) and C–H⋯S angles of 141.9(2) (2) and 143.5(1)° (3) to form endless double chains (Fig. 3). Interestingly, the bond lengths within the CS2 and CN2 units of the coordinated and free betaine show no significant differences (Table 1). Only the S–C–S bond angles contract from 130.7(1)° to 124.1(2)° in 2 and to 123.5(1)° in 3. Furthermore, the CS2/CN2 planes are now nearly orthogonally arranged with dihedral angles of 87.1° in both coordination polymers.
with Cu(I) atoms adopting trigonal-planar coordination geometries within their S2X donor sets (mean valence angle 119.99° within 2 and 119.98° within 3; see Table 1). The Cu–S bonds are equal in length and average to 2.244 Å in both 2 and 3. Pairs of polymeric chains are linked via intermolecular Cbenzyl–H⋯S interactions with H⋯S distances of 2.84 (2) and 2.79 Å (3) and C–H⋯S angles of 141.9(2) (2) and 143.5(1)° (3) to form endless double chains (Fig. 3). Interestingly, the bond lengths within the CS2 and CN2 units of the coordinated and free betaine show no significant differences (Table 1). Only the S–C–S bond angles contract from 130.7(1)° to 124.1(2)° in 2 and to 123.5(1)° in 3. Furthermore, the CS2/CN2 planes are now nearly orthogonally arranged with dihedral angles of 87.1° in both coordination polymers.
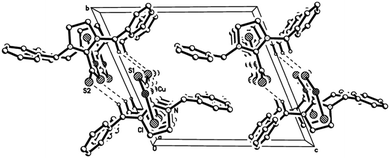 | ||
| Fig. 3 Crystal packing of 2 and 3 viewed along with intermolecular Cbenzyl–H⋯S contacts shown as dotted lines. H-atoms not involved in bonding are omitted. | ||
To the best of our knowledge, 2 and 3 are the first imidazolinium dithiocarboxylate copper complexes known so far. Coordination polymers with other dithiocarboxylates and copper are also not known. In this context, it should be mentioned that several mono-19 and polynuclear19h,20 copper dithiocarboxylate complexes as well as other dithiocarboxylate compounds with metals, such as gold,21 manganese,22 cadmium23 and mercury24 have been described.
The one-dimensional chain within crystals of 2 and 3 consisting of alternating Cu(I) and sp2 hybridized negatively charged CS2 units define an infinite 1-dimensional d–π system. Taking into account that copper containing 1-D-coordination polymers have shown potential as catalysts25 and have been applied as a photoactive material for dye-sensitized solar cells,26 it was decided to investigate the potential of the new material as a heterogeneous photocatalyst in case of a thiol–ene reaction. This reaction is of exceptional importance because of its wide spectrum of applications in synthetic sulphur chemistry.27 In a preliminary study, the addition of thiophenol to styrene was explored with white light (Scheme 3). This reaction has been, for example, catalysed with the photocatalyst Ru(bpz)32+ under homogenous conditions.28 However, the application of reusable heterogeneous systems based on low-cost and better biocompatible metals would be beneficial.
The existence of 2 and 3 is restricted to the crystalline state. Any attempts to dissolve the compounds are expected to result in depolymerisation reactions. ES-MS and NMR analysis (see ESI Fig. S1–S4†) of solutions obtained from reactions of 2 and 3 with dimethylsulfoxide or methanol confirm the formation of the mononuclear complexes [Cu(Bn2ImCS2)2]+[CuX2]− (X = Cl, Br). This observation supports our assumption that a breakup of the 1-D-coordination polymer takes place upon solvent attack. The stability of 2 and 3 towards acetonitrile allowed for probing the crystals as heterogeneous catalyst. In the case of 2, the formation of the photoproduct in 60% yield after 40 h was observed. A control reaction in the absence of light gave a yield of 23%, while under light conditions in the absence of crystalline 2 a yield of 34% was found. When the reaction was carried out with a solution obtained from 2 and dichloromethane, no catalytic activity was found, again suggesting that the electronic properties of the intact copper coordination polymer in the solid state are essential for the catalytic activity. Compound 3 showed no activity. A difference in the catalytic activity of different halide ligands on transition metal complexes is rare but not unknown, and cannot always be predicted.29 Although the catalytic activity of compound 2 is moderate, this is the first example of a coordination polymer containing copper suited to be a heterogeneous photo-redox catalyst.
Conclusions
In conclusion, an imidazolinium-dithiocarboxylate has been applied for the first time as a ligand towards copper(I) halides. The resulting 1-D-coordination polymers 2 and 3 show a linear structure with {CuX} units attached to negatively charged CS2 donor groups of the betaine resulting in an infinite d–π system. The potential of the material as a heterogeneous photocatalyst was shown in a radical thiol–ene reaction for the first time. Presently, we investigate the catalytic activity of our material with other thiols and try to optimize the reaction conditions also by using other imidazolinium-dithiocarboxylates. Besides investigations directed towards regioselective photoredox reactions, the use as dyes in TiO2 based photovoltaic cells is explored as well.Acknowledgements
We would like to thank the German research council (DFG) and the German Federal Ministry of education and research (BMBF) for the continuous support of our work. D. Konieczna and J. Ortmeyer thank the Evonik Foundation and Fonds der Chemischen Industrie (FCI) for granting doctoral fellowships each.Notes and references
- (a) W. Kirmse, Eur. J. Org. Chem., 2005, 237 CrossRef CAS; (b) L. Delaude, Eur. J. Inorg. Chem., 2009, 1681 CrossRef CAS.
- (a) J. Nakayama, J. Synth. Org. Chem., Jpn., 2002, 60, 106 CrossRef CAS; (b) Sulfur Lett., 1993, 15, 239 Search PubMed.
- H. E. Winberg and D. D. Coffman, J. Am. Chem. Soc., 1965, 87, 2776 CrossRef CAS.
- (a) W. Schössler and M. Regitz, Chem. Ber., 1974, 104, 1931 CrossRef; (b) W. Krasuski, D. Nikolaus and M. Regitz, Liebigs Ann. Chem., 1982, 1451 CrossRef CAS; (c) W. S. Sheldrick, A. Schönberg, E. Singer and E. Eckert, Chem. Ber., 1980, 113, 3605 CrossRef CAS; (d) D. H. Clemens, A. J. Bell and J. L. O'Brien, Tetrahedron Lett., 1965, 6, 3257 CrossRef; (e) N. Kuhn, H. Bohnen and G. Henkel, Z. Naturforsch., 1994, 49b, 1473 Search PubMed; (f) J. Nakayma and I. Akiyma, J. Chem. Soc., Chem. Commun., 1992, 1522 RSC; (g) A. Nagasawa, I. Akiyama, S. Mashima and J. Nakayama, Heteroat. Chem., 1995, 6, 45 CrossRef CAS; (h) K. Akimoto and J. Nakayama, Heteroat. Chem., 1997, 8, 505 CrossRef CAS; (i) L. L. Borer, J. V. Kong and D. E. Oram, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1989, 45, 1169 CrossRef; (j) T. Fujihara, T. Ohba, A. Nagasawa, J. Nakayama and K. Yoza, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2002, 58, o558 Search PubMed; (k) J. Nakayama, T. Otani, T. Tadokoro, Y. Sugihara and A. Ishii, Chem. Lett., 2001, 768 CrossRef CAS; (l) T. Otani, Y. Sugihara, A. Ishii and J. Nakayama, Heteroat. Chem., 1998, 9, 703 CrossRef CAS; (m) N. Kuhn, G. Weyers and G. Henkel, Chem. Commun., 1997, 627 RSC; (n) N. Kuhn, G. Weyers, S. Dummling and B. Speiser, Phosphorus, Sulfur Silicon Relat. Elem., 1997, 128, 45 CrossRef CAS; (o) S. Dummling, B. Speiser, N. Kuhn and G. Weyers, Acta Chem. Scand., 1999, 53, 876 CrossRef CAS PubMed; (p) J. Nakayama, T. Otani, Y. Sugihara and A. Ishii, Chem. Lett., 1998, 321 CrossRef CAS; (q) J. Nakayama, L. Zhao, T. Otani, Y. Sugihara and A. Ishii, Chem. Lett., 2000, 1248 CrossRef CAS.
- (a) M. L. Ziegler, H. Weber, B. Nuber and O. Serhadle, Z. Naturforsch., B: J. Chem. Sci., 1987, 42b, 1411 Search PubMed; (b) M. L. Ziegler, K. Blechschmitt, H. Bock, E. Guggolz and R. P. Korswagen, Z. Naturforsch., B: J. Chem. Sci., 1988, 43, 590 CAS; (c) S. R. Banerjee, A. Nagasawa and J. Zubieta, Inorg. Chim. Acta, 2002, 340, 155 CrossRef CAS; (d) L. L. Borer, J. V. Kong, P. A. Keihl and D. M. Forkey, Inorg. Chim. Acta, 1987, 129, 223 CrossRef CAS; (e) L. L. Borer, J. Kong and E. Sinn, Inorg. Chim. Acta, 1986, 122, 145 CrossRef CAS; (f) I. Miyashita, K. Matsumoto, M. Kobayashi, A. Nagasawa and J. Nakayama, Inorg. Chim. Acta, 1998, 283, 256 CrossRef CAS; (g) U. Siemeling, H. Memczak, C. Bruhn, F. Vogel, F. Traeger, J. E. Baio and T. Weidner, Dalton Trans., 2012, 41, 2986 RSC; (h) S. Naeem, L. Delaude, A. J. P. White and J. D. E. T. Wilton-Ely, Inorg. Chem., 2010, 49, 1784 CrossRef CAS PubMed; (i) S. Naeem, A. L. Thompson, A. J. P. White, L. Delaude and W. E. Lionel, Dalton Trans., 2011, 40, 3737 RSC; (j) T. Sugaya, T. Fujihara, A. Nagasawa and K. Unoura, Inorg. Chim. Acta, 2009, 362, 4813 CrossRef CAS PubMed.
- (a) T. Sugaya, T. Ohba, F. Sai, S. Mashima, T. Fujihara, K. Unoura and A. Nagasawa, Organometallics, 2013, 32, 3441 CrossRef CAS; (b) L. Delaude, X. Sauvage, A. Demonceau and J. Wouters, Organometallics, 2009, 28, 4056 CrossRef CAS; (c) M. J. D. Champion, R. Solanki, L. Delaude, A. J. P. White and J. D. E. T. Wilton-Ely, Dalton Trans., 2012, 41, 12386 RSC.
- L. Delaude, A. Demonceau and J. Wouters, Eur. J. Inorg. Chem., 2009, 1882 CrossRef CAS.
- A. Blanrue and R. Wilhelm, Synlett, 2004, 2621 CAS.
- O. Sereda, A. Blanrue and R. Wilhelm, Chem. Commun., 2009, 1040 RSC.
- A. Blanrue and R. Wilhelm, Synthesis, 2009, 583 CAS.
- D. D. Konieczna, A. Blanrue and R. Wilhelm, Z. Naturforsch., 2014, 69b, 596 CrossRef.
- S.-Y. Zhang, Z. Zhang and M. J. Zaworotko, Chem. Commun., 2013, 49, 9700 RSC.
- (a) D. Maspoch, D. Ruiz-Molina and J. Veciana, J. Mater. Chem., 2004, 14, 2713 RSC; (b) C. Zhang, Y. Chen, H. Ma, T. Yu, B. Liu and H. Pang, New J. Chem., 2013, 37, 1364 RSC; (c) X. Wang, X.-B. Li, R.-H. Yan, Y.-Q. Wang and E.-Q. Gao, Dalton Trans., 2013, 42, 10000 RSC; (d) P. Amo-Ochoa, O. Castillo and F. Zamora, Dalton Trans., 2013, 42, 13453 RSC.
- (a) A. Corma, H. García and F. X. L. i. Xamena, Chem. Rev., 2010, 110, 4606 CrossRef CAS PubMed; (b) D. Farrusseng, S. Aguado and C. Pinel, Angew. Chem., Int. Ed., 2009, 48, 7502 CrossRef CAS PubMed; (c) J. Lee, O. F. Fartha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC; (d) J. Garcia-Álvarez, J. Díez, C. Vidal and C. Vicent, Inorg. Chem., 2013, 52, 6533 CrossRef PubMed.
- (a) L. J. Murray, M. Dincá and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC; (b) J. L. C. Rowsell and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4670 CrossRef CAS PubMed; (c) B. Chen, S. Xiang and G. Qian, Acc. Chem. Res., 2010, 43, 1115 CrossRef CAS PubMed.
- M. A. Green, Nat. Mater., 2010, 9, 539 CrossRef CAS PubMed.
- R. Siebert, A. Winter, M. Schmitt, J. Popp, U. S. Schubert and B. Dietzek, Macromol. Rapid Commun., 2012, 33, 481 CrossRef CAS PubMed.
- N. Kuhn, E. Niquet, M. Steinmann and I. Walker, Z. Naturforsch., 1999, 54b, 1181 Search PubMed.
- (a) T. C. Deivaraj and J. J. Vittal, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2001, 57, m566 CAS; (b) D. F. Zigler, E. Tordin, G. Wu, A. Iretski, E. Cariati and P. C. Ford, Inorg. Chim. Acta, 2011, 374, 261 CrossRef CAS PubMed; (c) I. Garcia-Orozco, J. G. Lopez-Cortes, M. C. Ortega-Alfaro, R. A. Toscano, G. Penieres-Carrillo and C. Alvarez-Toledano, Inorg. Chem., 2004, 43, 8572 CrossRef CAS PubMed; (d) H. Otto and H. Werner, Chem. Ber., 1987, 120, 97 CrossRef CAS; (e) M. Pellei, S. Alidori, M. Camalli, G. Campi, G. G. Lobbia, M. Mancini, G. Papini, R. Spagna and C. Santini, Inorg. Chim. Acta, 2008, 361, 1456 CrossRef CAS PubMed; (f) M. Kondo, E. Shimizu, T. Horiba, H. Tanaka, Y. Fuwa, K. Nabari, K. Unoura, T. Naito, K. Maeda and F. Uchida, Chem. Lett., 2003, 32, 944 CrossRef CAS; (g) A. Camus, N. Marsich and G. Nardin, J. Organomet. Chem., 1980, 188, 389 CrossRef CAS; (h) A. Camus, N. Marsich, A. M. M. Lanfredi and F. Ugozzoli, Inorg. Chim. Acta, 1989, 161, 87 CrossRef CAS; (i) P. Strauch, B. Dempe, R. Kempe, W. Dietzsch, J. Sieler and E. Hoyer, Z. Anorg. Allg. Chem., 1994, 620, 498 CrossRef CAS; (j) J. Vicente, P. Gonzalez-Herrero, Y. Garcia-Sanchez, P. G. Jones and D. Bautista, Eur. J. Inorg. Chem., 2006, 115 CrossRef CAS.
- (a) A. M. M. Lanfredi, F. Ugozzoli, A. Camus and N. Marsich, Inorg. Chim. Acta, 1985, 99, 111 CrossRef; (b) A. M. M. Lanfredi, A. Tiripicchio, A. Camus and N. Marsich, J. Chem. Soc., Dalton Trans., 1989, 753 RSC; (c) I. Bassanetti, M. Gennari, L. Marchio, M. Terenghi and L. Elviri, Inorg. Chem., 2010, 49, 7007 CrossRef CAS PubMed; (d) M. Biagini-Cingi, A. M. M. Lanfredi, F. Ugozzoli, A. Camus and N. Marsich, Inorg. Chim. Acta, 1997, 262, 69 CrossRef CAS; (e) D. Coucouvanis, D. Swenson, N. C. Baenziger, R. Pedelty and M. L. Caffery, J. Am. Chem. Soc., 1977, 99, 8097 CrossRef CAS; (f) D. Coucouvanis, D. Swenson, N. C. Baenziger, R. Pedelty, M. L. Caffery and S. Kanodia, Inorg. Chem., 1989, 28, 2829 CrossRef CAS.
- M.-R. Azani, O. Castillo, M. L. Gallego, T. Parella, G. Aullón, O. Crespo, A. Laguna, S. Alvarez, R. Mas-Ballesté and F. Zamora, Chem.–Eur. J., 2012, 18, 9965 CrossRef CAS PubMed.
- M. Ciampolini, C. Mengozzi and P. Orioli, J. Chem. Soc., Dalton Trans., 1975, 2051 RSC.
- V. G. Young and E. R. T. Tiekink, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2002, 58, m537 CAS.
- A. M. Bond, R. Colton, A. F. Hollenkamp, B. F. Hoskins and K. McGregor, J. Am. Chem. Soc., 1987, 109, 1969 CrossRef CAS.
- J. García-Álvarez, J. Díez, J. Gimeno, F. J. Suárez and C. Vincent, Eur. J. Inorg. Chem., 2012, 5854 CrossRef.
- N. Tanaka, T. Okubo, H. Anma, K. H. Kim, Y. Inuzuka, M. Maekawa and T. Kuroda-Sowa, Eur. J. Inorg. Chem., 2013, 3384 CrossRef CAS.
- A. Gress, A. Volkel and H. Schlaad, Macromolecules, 2007, 40, 7928 CrossRef CAS.
- (a) E. L. Tyson, M. S. Ament and T. P. Yoon, J. Org. Chem., 2013, 78, 2046 CrossRef CAS PubMed; (b) M. H. Keylor, J. E. Park, C.-J. Wallentin and C. R. J. Stephenson, Tetrahedron, 2014, 70, 4264 CrossRef CAS PubMed; (c) E. L. Tyson, Z. L. Niemeyer and T. P. Yoon, J. Org. Chem., 2014, 79, 1427 CrossRef CAS PubMed.
- (a) K. Fagnou and M. Lautens, Angew. Chem., Int. Ed., 2002, 41, 26 CrossRef CAS; (b) S. L. Zhang and Y. Q. Ding, Organometallics, 2011, 30, 633 CrossRef CAS; (c) L. W. Mapson, Biochem. J., 1945, 39, 228 CAS; (d) S.-L. Zhang, L. Liu, Y. Fu and Q.-X. Guo, Organometallics, 2007, 26, 4546 CrossRef CAS; (e) W. Mo, H. Xiong, J. Hu, Y. Ni and G. Li, Appl. Organomet. Chem., 2010, 24, 576 CrossRef CAS; (f) A. Jutand, Appl. Organomet. Chem., 2004, 18, 574 CrossRef CAS; (g) P. A. Evans, D. K. Leahy and L. M. Slieker, Tetrahedron: Asymmetry, 2003, 14, 3613 CrossRef CAS PubMed; (h) P. A. Evans and D. K. Leahy, J. Am. Chem. Soc., 2002, 124, 7882 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. Anisotropic displacement ellipsoids are drawn at the 50% probability level. For experimental procedures, characterization data and crystallographic details (CCDC 1008787, 1000389 and 1000390), see DOI: 10.1039/c4ra09033k |
| This journal is © The Royal Society of Chemistry 2015 |