A novel cementitious microfiltration membrane: mechanisms of pore formation and properties for water permeation†
Zhe Wang,
Zhonglin Chen,
Jing Chang,
Jimin Shen*,
Jing Kang,
Lei Yang and
Qian Chen
State Key Laboratory of Urban Water Resource and Environment, School of Municipal & Environmental Engineering, Harbin Institute of Technology, Harbin 150090, China. E-mail: shenjimin@hit.edu.cn; Fax: +86-451-86283028; Tel: +86-451-86287000
First published on 21st November 2014
Abstract
This study involved the fabrication of a novel low-cost microfiltration membrane using quartz and cement. The entire fabrication process was conducted at room temperature, rather than utilizing high-temperature sintering, as is generally done during the fabrication of traditional inorganic membranes. The mean pore size of the membrane ranged from 0.4 to 2.4 μm depending on the quartz-to-cement ratio (q/c) used. Two types of pores (I and II) were formed in the membrane. The formation of type I pores was mainly attributed to the stacking of cement particles. The formation of type II pores, which were less prevalent than type I pores, was attributed to the division of bigger pores by the thin needle-like ettringite. The sizes of the type I and type II pores varied significantly, depending on the used q/c. Moreover, investigation of the membrane properties showed that as the q/c used increased, increases were obtained for both the membrane porosity (from 18.4% to 31.4%) and water flux (from 0.16 to 13.26 m3 m−2 h−1 bar−1), whereas the bending strength decreased (from 9.75 to 3.54 MPa). Additionally, element dissolution experiments demonstrated that the membrane was suitable for use for water treatment.
Introduction
The World Health Organization estimates that 1 billion people currently do not have access to clean, piped water.1 The challenge of providing acceptable drinking water is further expanded by population growth, industrialization, the contamination of available freshwater resources and climate change.2 In developing countries, this situation is severe, and the development of a cheap and efficient water treatment process is particularly valuable.In the water treatment process, microfiltration has been used broadly because of its low cost, high flux and simpler production procedures.3–6 According to the membrane materials used, microfiltration membranes can be divided into two categories: organic and inorganic membranes. Compared to organic membranes, inorganic membranes have many advantages, such as chemical stability (e.g., ozone resistance), pressure resistance, insensitivity to bacterial action, steam sterilizability and a longer lifetime.7,8 Therefore, inorganic membranes have gradually attracted the attention of researchers and have been applied to purify contaminated water.9–11 Furthermore, the inorganic microfiltration membrane can be used as a pretreatment in the water desalination process, and it can also be used as a support membrane for the fabrication of inorganic ultrafiltration and nanofiltration membrane. However, the current commercial inorganic membranes are not suitable for large-scale water treatment processes because of their high cost, which is a result of the use of expensive raw materials (e.g., alumina, zirconia and titania) and the high sintering temperature required.12 To address this issue, some low-cost raw materials, such as kaolin,13–15 fly ash,16 clay,17–19 mullite20,21 and natural zeolite,22 have been progressively used to fabricate inorganic membranes. However, the development of technologies to avoid the sintering process during inorganic membrane production has received little attention in recent studies.
Portland cement is commonly used in building and is characterized by good strength. Cement contains many impure chemical constituents, most of which are tricalcium (C3S), dicalcium silicate (β-C2S), tricalcium aluminate (C3A) and alumino-ferrite phases (C4AF).23,24 These compounds can react with water under ambient conditions and produce relatively insoluble hydration products. Based on the above, the fabrication of a new type of inorganic membrane under ambient conditions using low-cost cement is valuable. However, hydrated cement has worse water permeability than traditional inorganic microfiltration membrane due to the limited porosity and small pore size of the former.25 To solve the problem, many cement-based materials have been used to permeate water in the engineering applications. For example, larger aggregates were mixed with smaller cement particles to produce a pervious pavement, which was used to transport large volumes of water through the material structure to eliminate the problems of storm-water runoff and rainwater acidification.26–28 Therefore, in this study, the uniformly larger quartz particles were added to the system of cement and water. The quartz particles could disrupt the growth of the cement hydration products and thereby increase the porosity and pore size of this novel cementitious membrane.
In this study, we reported a novel low-cost microfiltration membrane that was fabricated with cement and quartz at room temperature. The membranes were fabricated with various quartz-to-cement ratios (q/c), and the resulting microstructures were investigated to explain the mechanisms of the formation of membrane pores. Furthermore, the permeability, bending strength and element stability of the membrane were also investigated to verify its potential for various applications.
Experimental
Raw materials
Quartz (≥99.0%, Xi Long Chemical Co., Ltd., China) and Portland cement (PO 42.5, Tian E®, China) were used as the raw materials for the fabrication of the cementitious microfiltration membrane. Chemical composition of the Portland cement used in this research was listed in the ESI (Table S1†) to facilitate the reproducibility of this membrane.Membrane fabrication
The membranes were produced using quartz, cement and deionized water. The quartz-to-cement weight ratios (q/c) used ranged from 0.3 to 4.0, as shown in Table 1. Fabrication of the cementitious membrane was based on a dry press molding process. First, the quartz particles were added to the cement particles, followed by dry-mixing for 2 min (10 r s−1) in a stainless-steel agitator. An appropriate amount of deionized water was then introduced into the mixture with stronger mechanical stirring (30 r s−1) to prepare a paste. Next, 10.5 g of the paste was placed into a circular stainless-steel mold consisting of a ring (70 mm outer diameter, 50 mm inner diameter and 3 mm thickness) and a desk (70 mm diameter and 3 mm thickness). A thin plastic sheet was sandwiched between the ring and the desk to facilitate separation of the paste from the mold. The mold was then carefully transported to a pressure shaping machine, and the paste was pressed at 6 MP for 1 min. Next, the mold was placed in a standard curing box (Shang Hai Jing Hong Laboratory Instrument Co., Ltd., China), in which the temperature and humidity were controlled at 20 °C and 95%, respectively. After 48 h, the shaped paste was taken out of the mold and stored in the standard curing box again for 10 days. Ten days later, the cementitious microfiltration membrane was formed, with a diameter of 50 mm and a thickness of 3 mm. Finally, the membrane was polished by abrasive paper to ensure that it was smooth and flat.| Quartz to cement ratio (q/c) | Quartz (g) | Cement (g) | Demonized water (mL) | |
|---|---|---|---|---|
| a | 0.3 | 10.0 | 30.0 | 7.0 |
| b | 0.5 | 13.3 | 26.7 | 7.0 |
| c | 1.0 | 20.0 | 20.0 | 7.0 |
| d | 2.0 | 26.7 | 13.3 | 7.0 |
| e | 3.0 | 30.0 | 10.0 | 7.0 |
| f | 4.0 | 32.0 | 8.0 | 7.0 |
Characterization methods
The phase composition of the raw materials was identified by X-ray diffraction (XRD), which was carried out on a Rigaku D/max-rA diffractometer with Cu Kα radiation (λ = 1.5405 Å). The particle size distributions of the raw materials were measured by dynamic light scattering using a Mastersizer 2000 apparatus (Malvern Instrument Co., Ltd., UK).The surface and cross-section morphologies of the membranes were characterized by scanning electron microscopy (SEM, Quanta 200FEG, FEI Corp., USA) to observe the connection between the quartz and cement particles.
The pore size and distribution of the membranes were measured using a pore size distribution analyzer (PSDA) (Nan Jing Gao Qian Functional Materials Co., Ltd., China) according to the gas–liquid displacement method.29 The PSDA could measure the diameters of the opened pores in the membrane in the range of 0.1 to 100 μm using a precise mass flow meter and a gaseous pressure sensor. The accuracy and stability of the PSDA was verified by measuring three membranes that were produced under the same condition. The results were listed in Fig. S1.†
The porosity of the membrane was measured according to the Archimedes method30 using water as the immersing medium, as specified in ASTM C20-00. All the porosities measured in this study were the open porosity of the membrane.
The elemental composition of the membrane was tested using an X-ray fluorescence spectrometer (XRF, AXIOS-PW 4400, PANalytical, Netherlands).
The water permeation experiments were carried out using a laboratory-made setup (Fig. 1). The setup consisted of a cylindrical container (stainless steel) for storing water, a base (stainless steel) for loading the membrane and a Teflon packing plate for sealing. The entire setup was fastened to the sample using a stainless-steel clip. A nitrogen cylinder was connected to the inlet of the cylindrical container to pressurize the water (deionized water, 25 °C). The water permeation tests involved the measurement of the permeated liquid volume within 10 min under various trans-membrane pressure drops (40–160 kPa) that were typically used in microfiltration. The practical permeation area of the membrane was 7 cm2. The experiments were repeated three times.
The bending strength of the membrane was tested by the three point bending method15 using a universal testing machine (Instron 5569, Instron Corporation, U.S.), with a span length of 30 mm. The samples were 3 mm × 4 mm × 40 mm rectangular bars.
An atomic emission spectrometer (ICP-AES, Optima 5300DV, Perkin Elmer, U.S.) was used to detect the elements in the membrane effluent to access the safety of the membrane materials.
Results and discussion
Characterization of the raw materials
The XRD spectra of cement and quartz particles are presented in Fig. 2a and b. Fig. 2a shows that the cement contains multiple man-made chemical constituents, including Ca3SiO5, Ca2SiO4, Ca3Al2O6, Ca2AlFeO5 and SiO2, as the main composition of the ordinary Portland cement.24 The XRD spectrum of the quartz (Fig. 2b) is in agreement with the JCPDS database file (PDF# 99-0088) which indicates that the quartz used in the work is ideal quartz (SiO2). The particle size distributions of the quartz and cement are shown in Fig. 2c. Quartz particles in the range of 28.6 to 81.5 μm (D50 = 50.0 μm) and cement particles in the range of 3.0 to 33.4 μm (D50 = 14.6 μm) are obtained by screening. In the membrane, the quartz can act as a support and the cement can act as a binder, filling in the gaps between the quartz particles. Deionized water is used to induce the hydration reaction of the cement. | ||
| Fig. 2 Raw materials analysis. (a) XRD patterns of the cement, (b) XRD patterns of the quartz and (c) particle size distributions of the quartz and cement used. | ||
Microstructure observation and pore characterization of the membrane
The microstructures of the membranes are investigated to observe the stacking of raw particles in the membrane. The results in Fig. 3a–e show the surface morphology of the novel membranes at different weight ratios of quartz-to-cement (q/c). In the membrane structures, the supporting function of the quartz particles gradually increases as the used q/c increased, and the smaller cement particles are embedded on the surface of the quartz particles and facilitate binding. Furthermore, Fig. 3 shows that a similar stacking structure of particles is observed for the cementitious membrane and the conventional sintered membrane (a Al2O3/TiO2 membrane).31 This may be because the larger-sized quartz and Al2O3 particles play a similar supporting function and the smaller-sized cement and TiO2 particles act as the source of binding phase in the respective membranes. In the traditional sintered Al2O3/TiO2 membrane, TiO2 with smaller size order is normally used as a sintering promoter agent for the reason that titania can react with alumina to form aluminum titanate (Al2TiO5) through solid-state reactions (above 1280 °C) at necks between the larger-sized alumina particles.31 Therefore, in this sintered membrane, the pores form from the stacking of alumina particles by the newly formed Al2TiO5 phase. Analogously, in the novel cementitious membrane, the larger-sized quartz can be recommended as the membrane support which partly replaces the above function of alumina (besides being a support, the alumina also participates in the solid-state reaction), and the smaller sized cement plays a binding function, similar to the aforementioned Al2TiO5 phase. However, the cementitious membrane can be fabricated under ambient conditions, unlike the sintered Al2O3/TiO2 membrane. | ||
| Fig. 3 SEM images (×1000) of the cementitious membrane surface. The membranes are produced at different q/c values: (a) 0.3, (b) 1.0, (c) 2.0, (d) 3.0 and (e) 4.0. | ||
The pore size distributions of the membranes are studied, as shown in Fig. 4. The results indicate that the membrane pores are mainly single micrometers in size (<8 μm) with a narrow size distribution, which results from the narrow particle size distribution of the raw materials. Moreover, with the increase in the q/c used in the membrane, the pore size distribution changes significantly from a unimodal distribution to a bimodal distribution. It is possible that a new type of pores forms in the membrane at higher q/c. The results in Fig. 5 show that the mean pore size of the membranes increases significantly from 0.4 μm to 2.4 μm as the q/c increases from 0.3 to 4.0. Over the same q/c range, the membrane porosity also increases from 18.4% to 31.4%. Therefore, increasing the percentage of quartz in the raw materials can significantly improve the pore size and porosity of the novel membrane.
 | ||
| Fig. 4 Pore size distribution of the membranes fabricated at different q/c values: (a) 0.3, (b) 0.5, (c) 1.0, (d) 2.0, (e) 3.0 and (f) 4.0. | ||
Pore formation mechanisms of the membrane
The gas–liquid displacement method and SEM can measure the pore size of inorganic microfiltration membranes. Therefore, the results of these two methods shall be authenticated reciprocally. For example, in the traditional Al2O3/TiO2 membrane,31 the pore size measured by the gas–liquid displacement method is 6.8 μm, similar to the pore size shown in its SEM image. Because the cementitious membrane has a similar particle stacking structure compared with the traditional sintered membrane, which is mentioned above. Therefore, the particle stacking mold is also the most likely mechanism of the pore formation for the cementitious membrane. However, the pore size of the cementitious membrane detected by the SEM images (Fig. 3b–e, pore size > 10.0 μm) differs significantly from that detected by the pore size analyzer (Fig. 4c–f, 1.0–2.5 μm), especially when using a high q/c to fabricate the membrane. The differences in the pore sizes detected by the SEM images and the pore size analyzer indicate that the pore formation mechanisms of the cementitious membrane may have a certain particularity compared with the traditional membrane. The macro-pores from the larger-sized quartz stacking (shown in Fig. 3) are not the primary pores of the cementitious membrane.To investigate the pore formation mechanisms of the novel membrane, the relationship between the pore size of the membrane and the particle size of the raw materials is studied based on the supposition that the pores of the novel membrane are also formed from the stacking of particles. Some researchers previously reported the following relationship between the pore diameter and the particle diameter:
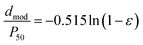 | (1) |
In eqn (1), “dmod” is the modal equivalent pore diameter, “P50” is the mean particle diameter and “ε” is the porosity of the membrane.32 When applied to the cementitious membrane studied here, using the average value of ε in Fig. 5 (25.3%), eqn (1) can be rewritten as follows:
| dmean = 0.150Dmean | (2) |
Fig. 2c shows that the Dmean of the cement particles is 14.6 μm and the Dmean of the quartz particles is 50.0 μm. Inserting the Dmean of the cement into eqn (2), the dmean of the cement is 2.2 μm, which represents the size of pores resulting from the cement particle stacking. This result is close to the membrane pore size, especially for the membrane produced at high q/c (Fig. 4e–f). Moreover, this value is only based on a cement particle stacking mode. At a low q/c (a relative large amount of cement), more cement hydration products will form and fill the cement stacking pores to decrease the membrane pore size. Therefore, if the growth of the cement hydration products on the surface of the cement particles is considered, the pore size shall be smaller, which produces a value closer to the mean pore size of the membrane fabricated at low q/c (Fig. 4a and b, only about 0.5 μm). In the same way, inserting the Dmean of the quartz into eqn (3), the dmean of the quartz is 7.5 μm, which represents the size of pores resulting from quartz particle stacking. This result is consistent with the largest-sized pores (6.5–8.0 μm) in every pore size distribution curve in Fig. 4. However, these macro-pores only occupy a small proportion of the total pores. Based on the above findings, it is possible that most of the opened pores in the cementitious membrane result from the stacking of the cement particles. The growth of the cement hydration products can decrease the size of pores, and this function is more obviously when increasing the amount of cement used.
To confirm the above pore-formation supposition, the connection part between the cement particles in the membrane are observed by SEM (using a high magnification of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), and a convincing result is obtained (Fig. 6a–c). The SEM images reveal that the pores form in the connections of the cement particles. The pore sizes in Fig. 6b and c are close to the results in Fig. 4d and f, respectively. Therefore, the formation of most pores in the cementitious membrane results from the stacking and connection of the cement particles. Moreover, at low q/c (Fig. 6a), the cement hydration products grow densely and the membrane pores are closed. As q/c increases from 0.5 to 4.0, the cement hydration products become sparse, and the membrane pores gradually open (Fig. 6a–c). This result can be explained from two perspectives. On one hand, when the membrane is produced with a high q/c, such as in Fig. 6c, the water-to-cement ratio of the membrane is also high, as shown in Table 1. Under this condition, water can occupy much more space in the membrane in the initial membrane production process. During the cement hydration reaction, the water is gradually consumed. Therefore, the greater amount of space occupied by the water is gradually vacated, and a higher membrane porosity can be obtained. On the other hand, the contact area between the cement particles is lower when a high q/c is used; thus, the growth of the cement hydration products is unfavorable. Therefore, at high q/c, the pore size of membrane (1–2 μm, Fig. 4f and 6c) is closer to the result for the cement particle stacking mode (2.2 μm, as mentioned above), and the membrane pores are insufficiently filled by the hydrated products of cement. In contrast, in the membrane produced at low q/c (Fig. 6a), there is less space among cement particles, leading the cement hydration products to grow densely and fill much more space among the cement particles. Therefore, the membrane pores are filled sufficiently by the hydrated products in this type of membrane, and the membrane pore size (<0.5 μm, Fig. 4a) is significantly affected.
000), and a convincing result is obtained (Fig. 6a–c). The SEM images reveal that the pores form in the connections of the cement particles. The pore sizes in Fig. 6b and c are close to the results in Fig. 4d and f, respectively. Therefore, the formation of most pores in the cementitious membrane results from the stacking and connection of the cement particles. Moreover, at low q/c (Fig. 6a), the cement hydration products grow densely and the membrane pores are closed. As q/c increases from 0.5 to 4.0, the cement hydration products become sparse, and the membrane pores gradually open (Fig. 6a–c). This result can be explained from two perspectives. On one hand, when the membrane is produced with a high q/c, such as in Fig. 6c, the water-to-cement ratio of the membrane is also high, as shown in Table 1. Under this condition, water can occupy much more space in the membrane in the initial membrane production process. During the cement hydration reaction, the water is gradually consumed. Therefore, the greater amount of space occupied by the water is gradually vacated, and a higher membrane porosity can be obtained. On the other hand, the contact area between the cement particles is lower when a high q/c is used; thus, the growth of the cement hydration products is unfavorable. Therefore, at high q/c, the pore size of membrane (1–2 μm, Fig. 4f and 6c) is closer to the result for the cement particle stacking mode (2.2 μm, as mentioned above), and the membrane pores are insufficiently filled by the hydrated products of cement. In contrast, in the membrane produced at low q/c (Fig. 6a), there is less space among cement particles, leading the cement hydration products to grow densely and fill much more space among the cement particles. Therefore, the membrane pores are filled sufficiently by the hydrated products in this type of membrane, and the membrane pore size (<0.5 μm, Fig. 4a) is significantly affected.
Fig. 6d–f show that higher q/c values corresponds to the growth of more needle-like compounds in the membrane pores. At high q/c, these needles grow longer and cross one another, dividing the membrane pores (Fig. 6f), which may increase the proportion of smaller pores in the membrane. This explanation is supported by the PSDA results in Fig. 4, which indicate that with increasing q/c, a new peak appears and increases in intensity. This new peak corresponds to a smaller pore size than the prominent peak in the pore size distribution curve does. These newly formed pores may result from the needle-like compounds dividing the larger membrane pores. Moreover, as the q/c increases, these needle-like compounds become more expansive, and their dividing function is more prominent (Fig. 6f). To clarify the source and composition of these needle-like compounds, EDX analysis is carried out, and the results are shown in Fig. 7c and d. The testing area of the EDX analysis is marked with a red box in the corresponding SEM images (Fig. 7a: the needle-like structure; Fig. 7b: the adjacent flat area). The result shows that in the needle-like compound structure, the contents of Al, S and Fe increase by 2.15, 1.10 and 4.42 times, respectively, compared to the adjacent flat area. The elements Al and S are characteristic of the ettringite, which results from the reaction of the calcium aluminate and the artificially added calcium sulfate. The element Fe is also involved in the formation of ettringite.23,33–37 Moreover, the ettringite has been found to have a needle-like structure in many studies.23,34,38 Thus, it is believed that the needle-like structure observed in this study is ettringite.
Based on the above analysis, details about the reactive process for the membrane formation are summarized as: (1) dry mixing of materials; (2) wet mixing of materials; (3) shaping by dry pressing; (4) membrane curing. In the first step, the dry mixing of quartz and cement particles results in that the larger-sized quartz particles are surrounded by the smaller-sized cement particles. Then in the second step of wet mixing, water is added and occupies the space among the particles. The third step of dry pressing decreases the pore volume of the system, resulting in that the distances between quartz particles are closer. Therefore, the cement particles are much closer with each other. In the fourth step of membrane curing, cement hydration reaction occurs, and the cement particles react with water to form multiple membrane binding phases (Ca3SiO5·nH2O, Ca2SiO4·nH2O, Ca3Al2O6·nH2O and Ca2AlFeO5·nH2O, etc.). This reaction gradually consumes the water. Great amount of spaces occupied by the water is gradually vacated in this stage, which supplies spaces for the further growth of these binding phases. These binding phases have 2 vital functions. On one hand, they connect the cement particles together, which fixing the quartz particles in the membrane, and increasing the strength of the membrane. On the other hand, the cement particles, which stack among the quartz particles, are connected by these binding phases to form the membrane pores. Moreover, with increasing q/c in the membrane fabrication process, ettringite can significantly widen the pore size distribution of the membrane, as observed in Fig. 4.
Water permeation test
The membrane water flux J (m3 m−2 s−1) is affected by many factors and can be estimated according to the Hagen–Poiseuille equation:39
 | (3) |
 | (4) |
The novel microfiltration membranes that are fabricated at different q/c are subjected to the water permeation test using deionized water. Fig. 8 shows the changes in the water fluxes (J) of the membrane under a varying trans-membrane pressure drop (40–160 kPa). The slopes of these flux curves can represent the water permeability (Lh) of the membrane used. As shown in Fig. 8, the water flux of each membrane has an excellent linear relationship with the trans-membrane pressure drop, which is consistent with eqn (3). Moreover, the water permeability of the membrane increases markedly from 0.16 to 13.26 m3 m−2 h−1 bar−1 when the used q/c increases from 0.3 to 4.0. These results can be explained by the increase of the porosity and the mean pore size of the membrane, as shown in Fig. 5. Furthermore, according to eqn (4), the water permeability is directly proportional to the mean pore size and the porosity of the membrane. Therefore, the water fluxes of the membranes fabricated at the high q/c (Fig. 8d–f) increase more obviously than that of the membranes fabricated at the low q/c (Fig. 8a–c) when the trans-membrane pressure drop increases. Compared to some traditional inorganic microfiltration membranes,15,40 the cementitious microfiltration membranes fabricated at high q/c in this study (Fig. 8d–f) have a comparable water permeability, based on similar porosities and pore sizes. However, the porosity of the cementitious membrane is approximately 30%, which limits the increase of the membrane permeation flux, compared to some traditional inorganic membranes with high porosity (e.g., the aforementioned Al2O3/TiO2 membrane with a porosity of 35–45% and a water permeability of 25–40 m3 m−2 h−1 bar−1). The low cost of the cementitious membrane can possibly cover this shortage in the water treatment process.
 | ||
| Fig. 8 Water flux of the membranes fabricated at different q/c values: (a) 0.3, (b) 0.5, (c) 1.0, (d) 2.0, (e) 3.0 and (f) 4.0. The error bars indicate the standard deviation of the mean (n = 3). | ||
Mechanical strength analysis
The mechanical strength of the prepared membrane is tested using the three-point bending strength method. The bending strength of the membrane, shown in Fig. 9, decreases markedly from 9.75 to 3.54 MPa as the used q/c increases from 0.3 to 4.0. This result can be attributed to the decrease of the cement proportion and the increase of the porosity in the membrane. In this study, the bending strength of the membranes with available water permeability (such as the membranes tested in Fig. 8d–f) can reach approximately 5 MPa. However, this value is still substantially less than that of traditional sintered membranes15,41,42 (5–45 MPa, commonly). The sintered inorganic membrane is mainly designed for use in the field of chemical engineering, in which the membrane often works under high temperature and high pressure and thus requires the high strength. However, the cementitious membrane is intended for use in the water treatment process; as a result, the limited bending strength of this membrane is considered to be sufficient. | ||
| Fig. 9 Bending strength of the membranes fabricated at different q/c values. The error bars indicate the standard deviation of the mean (n = 3). | ||
Element dissolution tests
Cement is a multiphase composite material and contains many elements, some of which are heavy metals and are harmful for health when present in the water supply. The potential contamination of heavy metals is becoming a concern.43–45 X-ray fluorescence (XRF) spectrometry is used to identify the elemental composition of the membrane. The tested membrane is produced using a q/c of 3.0. The result is shown in Table 2, and it indicates that many of the elements existing in the membrane are limited in the national drinking water standard of China (GB5749-2006)46 and the drinking water standards of the U.S. Environmental Protection Agency (EPA).47 Therefore, it is necessary to test the element contents in the membrane effluent to ensure the safety of the cementitious membrane. The experiment is performed using two trans-membrane pressure drops (20 kPa and 60 kPa). The results in Table 2 show that the concentrations of almost all of the element contents in the membrane effluent are lower than the limits set by the two drinking water standards considered for both the high (60 kPa) and low (20 kPa) trans-membrane pressure drops. The concentrations of the element Al obtained from the high and low pressure drop effluents (0.09 and 0.13 mg L−1, respectively) exceed the maximum limit set by the EPA (0.05 mg L−1) but are lower than the limit set by GB5749-2006 of China (0.2 mg L−1). Other harmful metals, such as Cr, Mn, Cu, Zn and Pb are not detected in the effluent samples. The results verify the safety of this novel membrane material.| Elements | pH | Na | Mg | Al | Si | P | S | K | Ca | Cr | Mn | Fe | Cu | Zn | Sr | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XRF (%) | — | 0.69 | 0.12 | 0.61 | 19.97 | 0.02 | 1.40 | 0.68 | 7.57 | 0.01 | 0.02 | 0.58 | 0.01 | 0.02 | 0.02 | 0.01 |
| Influent (mg L−1) | 6.45 | 0.05 | 0.03 | — | 0.06 | 0.02 | 0.13 | 0.41 | 0.18 | — | — | — | — | — | — | — |
| Effluent (mg L−1) (60 kPa) | 6.80 | 0.28 | 0.07 | 0.09 | 0.30 | 0.01 | 1.48 | 0.59 | 6.19 | — | — | — | — | — | 0.01 | — |
| Effluent (mg L−1) (20 kPa) | 7.21 | 0.73 | 0.07 | 0.13 | 0.70 | 0.01 | 2.60 | 1.16 | 9.18 | — | — | 0.01 | — | — | 0.02 | — |
| GB5749-2006, China (mg L−1) | 6.5–8.5 | 200 | — | 0.2 | — | — | — | — | — | 0.05 | 0.1 | 0.3 | 1 | 1 | — | 0.01 |
| EPA, USA 822-R-06-013 (mg L−1) | 6.5–8.5 | — | — | 0.05–0.2 | — | — | — | — | — | 0.1 | 0.05 | 0.3 | 1 | 5 | — | 0 |
Conclusions
A novel cementitious microfiltration membrane was prepared for water treatment with different quartz-to-cement ratios (q/c). The mechanisms of membrane pore formation can be summarized as follows. (1) The formation of the largest-sized pores was mainly attributed to the stacking of quartz particles, and the pore size was 6–8 μm. This pore type was formed under every q/c used in this study, comprising a small proportion of all pores in the sample. (2) The more prevalent pores in the membrane resulted from the stacking of the cement particles. The sizes of these pores ranged from 0.3 to 2.5 μm depending on the used q/c. (3) The aforementioned dominant pores could be divided by thin needle-like ettringites, forming smaller pores. Furthermore, the membrane properties were also studied. With the increasing q/c used in the membrane, the membrane porosity and water flux were improved; however, the bending strength decreased significantly. The element dissolution tests proved that the concentrations of the elements in the membrane effluent were lower than the limits set by China's national drinking water standard. Therefore, the cementitious membrane is a safe material that can be used in the water treatment process.Acknowledgements
This work was supported by the Fund for Creative Research Groups of China (Grant no. 51121062), the State Key Laboratory of Urban Water Resource and Environment (Harbin Institute of Technology) (no. 2014DX02) and the National Important Items of Science and Technology for the Control and Treatment of Water Pollution (Grant no. 2014ZX07405002).References
- R. F. Service, Science, 2006, 313, 1088–1090 CrossRef CAS PubMed.
- M. Elimelech and W. A. Phillip, Science, 2011, 333, 712–717 CrossRef CAS PubMed.
- I. Jedidi, S. Khemakhem, A. Larbot and R. B. Amar, Ceram. Int., 2009, 35, 2747–2753 CrossRef CAS PubMed.
- G. Belfort, R. H. Davis and A. L. Zydney, J. Membr. Sci., 1994, 96, 1–58 CrossRef CAS.
- W. Yuan and A. L. Zydney, J. Membr. Sci., 1999, 157, 1–12 CrossRef CAS.
- Q. B. Chang, J. E. Zhou, Y. Q. Wang, J. Liang, X. Z. Zhang, S. Cerneaux, X. Wang, Z. W. Zhu and Y. C. Dong, J. Membr. Sci., 2014, 456, 128–133 CrossRef CAS PubMed.
- A. Larbot, J. P. Fabre, C. Guizard, L. Cot and J. Gillot, J. Am. Ceram. Soc., 1989, 72, 257–261 CrossRef CAS PubMed.
- B. S. Karnik, S. H. Davies, M. J. Baumann and S. J. Masten, Environ. Sci. Technol., 2005, 39, 7656–7661 CrossRef CAS.
- N. W. Gao, M. Li, W. H. Jing, Y. Q. Fan and N. P. Xu, J. Membr. Sci., 2011, 375, 276–283 CrossRef CAS PubMed.
- D. Vasanth, G. Pugazhenthi and R. Uppaluri, J. Membr. Sci., 2011, 379, 154–163 CrossRef CAS PubMed.
- B. Zhu, Y. X. Hu, S. Kennedy, N. Milne, G. Morris, W. Q. Jin, S. Gray and M. Duke, J. Membr. Sci., 2011, 378, 61–72 CrossRef CAS PubMed.
- Y. C. Dong, X. Y. Feng, D. H. Dong, S. L. Wang, J. K. Yang, H. F. Gao, X. Q. Liu and G. Y. Meng, J. Membr. Sci., 2007, 304, 65–75 CrossRef CAS PubMed.
- F. Bouzerara, A. Harabi, S. Achour and A. Larbot, J. Eur. Ceram. Soc., 2006, 26, 1663–1671 CrossRef CAS PubMed.
- B. K. Nandi, B. Das, R. Uppaluri and M. K. Purkait, J. Food Eng., 2009, 95, 597–605 CrossRef CAS PubMed.
- J. N. Zhou, X. Z. Zhang, Y. Q. Wang, A. Larbot and X. B. Hu, J. Porous Mater., 2010, 17, 1–9 CrossRef CAS.
- I. Jedidi, S. Saidi, S. Khmakem, A. Larbot, N. Elloumi-Ammar, A. Fourati, A. Charfi and R. Ben Amar, Arabian J. Chem., 2009, 2, 31–39 CrossRef PubMed.
- N. Saffaj, M. Persin, S. A. Younsi, A. Albizane, M. Cretin and A. Larbot, Appl. Clay Sci., 2006, 31, 110–119 CrossRef CAS PubMed.
- S. Khemakhem, A. Larbot and R. Ben Amar, Ceram. Int., 2009, 35, 55–61 CrossRef CAS PubMed.
- S. Jana, M. K. Purkait and K. Mohanty, Appl. Clay Sci., 2010, 47, 317–324 CrossRef CAS PubMed.
- G. L. Chen, H. Qi, W. H. Xing and N. P. Xu, J. Membr. Sci., 2008, 318, 38–44 CrossRef CAS PubMed.
- Y. C. Dong, B. Lin, K. Xie, S. L. Wang, H. P. Ding, D. R. Fang, X. Q. Liu and G. Y. Meng, J. Alloys Compd., 2009, 477, 350–356 CrossRef CAS PubMed.
- Y. C. Dong, S. F. Chen, X. B. Zhang, J. K. Yang, X. Q. Liu and G. Y. Meng, J. Membr. Sci., 2006, 281, 592–599 CrossRef CAS PubMed.
- L. Black, C. Breen, J. Yarwood, C. S. Deng, J. Phipps and G. Maitland, J. Mater. Chem., 2006, 16, 1263–1272 RSC.
- D. D. Double, A. Hellawell and S. J. Perry, Proc. R. Soc. London, Ser. A, 1978, 359, 435–451 CrossRef CAS.
- H. N. Atahan, O. N. Oktar and M. A. Tasdemir, Construct. Build. Mater., 2009, 23, 1196–1200 CrossRef PubMed.
- B. J. Putman and A. I. Neptune, Construct. Build. Mater., 2011, 25, 3480–3485 CrossRef PubMed.
- N. Neithalath, M. S. Sumanasooriya and O. Deo, Mater. Charact., 2010, 61, 802–813 CrossRef CAS PubMed.
- X. H. Kuang and J. Sansalone, Water Sci. Technol., 2011, 63, 2992–2998 CrossRef CAS.
- Y. H. Wang, Y. Zhang, X. Q. Liu and G. Y. Meng, J. Sol-Gel Sci. Technol., 2007, 41, 267–275 CrossRef CAS PubMed.
- S. Workneh and A. Shukla, J. Membr. Sci., 2008, 309, 189–195 CrossRef CAS PubMed.
- H. Qi, Y. Q. Fan, W. H. Xing and L. Winnubst, J. Eur. Ceram. Soc., 2010, 30, 1317–1325 CrossRef CAS PubMed.
- Y. B. P. Kwan, D. J. Stephenson and J. R. Alcock, J. Porous Mater., 2001, 8, 119–127 CrossRef CAS.
- Y. Shimada and J. F. Young, Adv. Cem. Res., 2001, 13, 77–81 CrossRef CAS.
- P. Chindaprasirt, S. Thaiwitcharoen, S. Kaewpirom and U. Rattanasak, Cem. Concr. Compos., 2013, 41, 24–28 CrossRef CAS PubMed.
- G. Moeschner, B. Lothenbach, F. Winnefeld, A. Ulrich, R. Figi and R. Kretzschmar, Cem. Concr. Res., 2009, 39, 482–489 CrossRef PubMed.
- N. Meller, C. Hall, A. C. Jupe, S. L. Colston, S. D. M. Jacques, P. Barnes and J. Phipps, J. Mater. Chem., 2004, 14, 428–435 RSC.
- G. Moeschner, B. Lothenbach, J. Rose, A. Ulrich, R. Figi and R. Kretzschmar, Geochim. Cosmochim. Acta, 2008, 72, 1–18 CrossRef CAS PubMed.
- E. Sakai, Y. Nikaido, T. Itoh and M. Daimon, Cem. Concr. Res., 2004, 34, 1669–1673 CrossRef CAS PubMed.
- M. C. Almandoz, J. Marchese, P. Pradanos, L. Palacio and A. Hernandez, J. Membr. Sci., 2004, 241, 95–103 CrossRef CAS PubMed.
- B. K. Nandi, R. Uppaluri and M. K. Purkait, Appl. Clay Sci., 2008, 42, 102–110 CrossRef CAS PubMed.
- F. Bouzerara, S. Boulanacer, A. Harabi, B. Boudaira, S. Achour and S. Condom, Phys. Procedia, 2009, 2, 1449–1453 CrossRef CAS PubMed.
- I. Jedidi, S. Khemakhem, S. Saidi, A. Larbot, N. Elloumi-Ammar, A. Fourati, A. Charfi, A. Ben Salah and R. Ben Amar, Powder Technol., 2011, 208, 427–432 CrossRef CAS PubMed.
- J. L. Zhang, J. G. Liu, C. Li, Y. F. Nie and Y. Y. Jin, Cem. Concr. Res., 2008, 38, 675–680 CrossRef CAS PubMed.
- H. A. van der Sloot, Cem. Concr. Res., 2000, 30, 1079–1096 CrossRef CAS.
- M. A. Trezza and A. N. Scian, J. Hazard. Mater., 2007, 147, 188–196 CrossRef CAS PubMed.
- GB5749-2006, Ministry of Health of the People's Republic of China and Standardization Administration of the People's Republic of China, Beijing, 2006.
- EPA 822-S-12-001, Office of Water, U.S. Environmental Protection Agency, Washington, DC, 2012.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08980d |
| This journal is © The Royal Society of Chemistry 2015 |




