Synthesis and characterization of biocompatible tigecycline imbibed electrospun poly ε-caprolactone urethane urea fibers
M. Sabitha and
Sheeja Rajiv*
Department of Chemistry, Anna University, Chennai, Tamilnadu, India-600025. E-mail: sheeja@annauniv.edu; Fax: +91 44 22200889; Tel: +91 44 22358658
First published on 1st December 2014
Abstract
A novel antibiotic wound dressing for infected wounds was prepared by immersing electrospun poly ε-caprolactone urethane urea (PEUU) microfibers in tigecycline solution. The fibers were characterized using ATR-FTIR, 1H NMR, TGA-DTA, DSC and XRD. In vitro release studies showed a burst followed by a sustained release of tigecycline. Bacterial inhibition tests confirmed that the sensitive tigecycline had its activity intact. The NIH 3T3 fibroblast cell attachment on microfibers was examined using DAPI fluorescence imaging and scanning electron microscopy. The present study confirmed the ease in preparation and the use of tigecycline loaded PEUU as promising antimicrobial scaffold for the treatment of skin and soft tissue infections. The significance and novelty of the work lies in the successful use of the scaffold as a bandage to prevent wound infections.
Introduction
Electrospinning technique involves uniaxial stretching of the viscoelastic polymer solution or melts under the presence of electrostatic forces.1,2 Electrospun scaffold mimics the natural tissue and interacts at the molecular level to regulate the regeneration of the damaged tissue. Porous fibrous scaffolds are an excellent biomaterial for drug delivery and wound healing applications.3,4 They provide an anchor for cell attachment, proliferation and differentiation. Though most biomaterials are biocompatible, they are rigid in nature. Electrospun poly ester urethane urea fibers are favourable for skin and soft tissue applications5,6 as it is produces highly elastic and flexible scaffolds. Polyurethanes are segmented block copolymers, composed of hard and soft segments. The poly ε-caprolactone diol soft segment has low glass transition temperature of −60 °C and is highly elastic by nature.7 Aliphatic diisocyanates are biocompatible and undergoes controlled degradation to non-cytotoxic decomposition products. Isophorone diisocyanate (IPDI) based polyurethanes are more resistant to UV radiations.8 Polyurethane wound dressings prepared with IPDI and a photosensitive drug are cured by exposure to UV light without the release of heat.9 They have good mechanical properties, chemical resistance and stability.10 The introduction of the amino groups into the polyester urethane backbone can enhance moisture absorption and improves surface energy.11 Polyurethanes are widely used for the making of bandages for wound healing applications due to their elasticity, fatigue resistance, amenability and for their tolerance to skin tissues. The thermoplastic polyurethanes (TPU) can promote moist wound healing, removes exudates and prevents the fluid and protein loss from the wound areas.12,13 Porous polyurethane fiber mats have excellent oxygen permeability and prevent wound desiccation.14 Semipermeable polyurethane films (OpSite, Tegaderm) and semipermeable non-adhesive Allevyn are polyurethane wound dressings.15 Electrospun polyurethane dressings increased rate of epithelisation in wounds and resulted in a well organised dermis.16 Wounds in diabetic mice was improved with electrospun Polyester urethane urea dressings.17 Functionally modified TPU fibers have the potential application as wound dressings.18–20Skin and soft tissue infections (SSTIs) are the inflammatory bacterial invasion of the epidermis, dermis and the subcutaneous tissues.21 Tigecycline is a glycylcycline antibiotic used for the treatment of the SSTIs as well as for the intra-abdominal infections.22 Many uncomplicated SSTIs can be resolved with the local care. Uncomplicated SSTIs include the superficial cellulitis, carbuncles, folliculitis, impetigo, furunculosis, simple abscesses and the minor wound infections.23 Staphylococcus aureus is the commonest bacteria affecting skin and soft tissues. Most of the Staphylococcus sp. infections have gained resistance against penicillin and methicillin due to their indiscriminate usage. Tigecycline is a tetracycline derivative which has 9-t-butyl glycylamido side chain that facilitates the broader spectrum of their activity. It inhibits the protein synthesis by binding to the 30S ribosomal subunits of the bacteria.24
Electrospinning of hydrophobic polymer with a hydrophilic drug causes the drug molecules to be present on or near the fiber surface25,26 that can eliminate the colonizing bacteria in that region before they can proliferate. Controlled release of the antibiotics is desirable for treating many sustained infections. Coaxial electrospinning can also attribute to a pathway for incorporating drugs inside the fiber core that will prolong the release rate of drugs.27 Hydrophilic tigecycline was dissolved in milli Q water and loaded into the PEUU fibrous scaffolds by the imbibitions method, in which a particular weight of PEUU fibrous scaffold was immersed into the drug solution and dried. This versatile process of loading the drug molecules into the scaffolds does not require an input of energy and enables the drug to retain their structure, composition and the antibacterial activity. Even though, electrospinning of the polymer and the drug in a single solvent could produce an ideal drug delivery fibrous scaffold, tigecycline loaded electrospun PEUU matrix by the imbibition method has the advantages of no drug loss by the electrospinning process and also retains the antibacterial activities for the repair of skin and soft tissues which are infected by multidrug resistant strains of bacteria.
Materials and methods
Materials
Polycaprolactone diol (average Mn ∼ 2000), tigecycline hydrate (98%, HPLC), putrescine, stannous octoate, Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from the Sigma-Aldrich (India). Isophorone diisocyanate was purchased from the Alfa Aesar. Methicillin resistant Staphylococcus aureus were isolated from the hospitalized patients; Escherichia coli and Klebsiella pneumonia was procured from the MTCC, IMTECH Chandigarh, India. Muller Hinton agar was purchased from the Himedia Pvt Limited, Mumbai, India. NIH 3T3 fibroblast cells were obtained from the National Centre for Cell Science (NCCS), Pune, India. DAPI (4′,6 diamidino-2-phenylindole) was purchased from Life Technologies. Chloroform and Methanol were purchased from the Sisco Research Laboratories Private Limited, India. The electrospinning unit adopted in the current study was designed and developed in the department.Synthesis of the polyester urethane urea (PEUU)
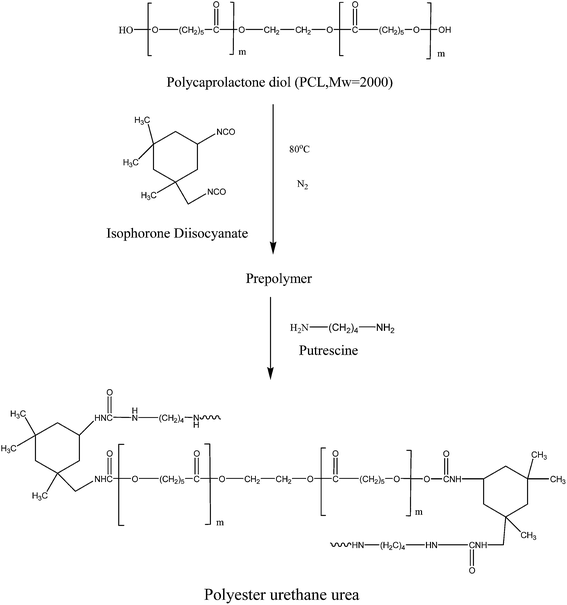
The biodegradable polyester urethane urea (PEUU) based on polycaprolactone diol, isophorone diisocyanate and putrescine was synthesized using a two-step polymerization method (Scheme 1). The mixture of isophorone diisocyanate and polycaprolactone diol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) was reacted with the catalyst at 80 °C for 4 h. IPDI was incorporated in the Scheme 1 due to its increased stability10 which could be suitable for its use as a scaffold for wound dressing. The prepolymer solution was cooled to room temperature, and the chain extender; putrescine was added drop wise with continuous stirring. The reaction was carried out at room temperature for about 18 h.28
1 molar ratio) was reacted with the catalyst at 80 °C for 4 h. IPDI was incorporated in the Scheme 1 due to its increased stability10 which could be suitable for its use as a scaffold for wound dressing. The prepolymer solution was cooled to room temperature, and the chain extender; putrescine was added drop wise with continuous stirring. The reaction was carried out at room temperature for about 18 h.28
Electrospinning of the polyester urethane urea (PEUU)
The PEUU polymer was dissolved in the chloroform and methanol solvents in the volume ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The addition of methanol in very small amounts was necessary to produce the uniformly oriented fibers. The solutions were prepared at room temperatures and gently stirred continuously for about 3–4 h. The PEUU solution at a concentration of 8 wt% was taken in a 5 ml syringe to which a needle tip of 0.55 mm diameter was attached. An electric potential of 20 kV was applied to the droplet at the tip of the syringe needle. The syringe pump was set to have a flow rate of 1 ml h−1. The distance between the tip of the needle and the aluminium metal collector was kept at 20 cm. The electrospinning process was carried out for approximately 4 h and the fibrous mats were later peeled from the aluminium foil collector.
1. The addition of methanol in very small amounts was necessary to produce the uniformly oriented fibers. The solutions were prepared at room temperatures and gently stirred continuously for about 3–4 h. The PEUU solution at a concentration of 8 wt% was taken in a 5 ml syringe to which a needle tip of 0.55 mm diameter was attached. An electric potential of 20 kV was applied to the droplet at the tip of the syringe needle. The syringe pump was set to have a flow rate of 1 ml h−1. The distance between the tip of the needle and the aluminium metal collector was kept at 20 cm. The electrospinning process was carried out for approximately 4 h and the fibrous mats were later peeled from the aluminium foil collector.
Preparation of tigecycline loaded electrospun PEUU (T-PEUU) mats
The electrospun PEUU mats weighing approximately 0.03 g were cut into the size of 1 × 1 cm. They were washed with de-ionized water. A second rinse with the de-ionized water was carried out and then they were dried in a biological safety cabinet followed by their sterilization using ultraviolet radiations. These sterilized mats were then kept in the Petri-dishes under the dark conditions. The stock concentration of tigecycline antibiotic was prepared by dissolving 10 mg of tigecycline hydrate in 15 ml of milli Q water (∼0.666 mg ml−1). From the stock concentrations, about 1.5, 1 and 0.5 ml (i.e., 1, 0.7 and 0.3 mg) were separately added to the sterilized 1 × 1 cm PEUU mats which are kept in the Petri dishes with the help of a dropper. These mats were kept in the dark and allowed to dry. The dried mats were characterized and investigated for antibacterial activity.Characterization methods
The Shimadzu RID-10A, LC-20AD model GPC instrument with a differential refractive index detector and two columns were used to determine the molecular weights of the synthesized PEUU polymer. The flow rate of the THF solvent was set to about 1 ml min−1 and the calibration of the GPC was performed using the polystyrene standards. The surface morphology of the fibers was examined using the FEI Quanta FEG 200-HRSEM scanning electron microscope. The samples were fractured with liquid nitrogen and were coated with a thin layer of gold before the analysis. ATR-FTIR spectroscopy was used to identify the functional groups present in the PEUU fibers and the functional groups of incorporated tigecycline in the PEUU electrospun matrix. ATR-IR spectra were carried out using PerkinElmer spectrophotometer equipped with a diamond crystal at an angle of incidence of 180°. Eight scans were performed and the spectra were recorded in the frequency range of 400–4000 cm−1. 1H NMR spectroscopy of the electrospun PEUU fibres was recorded by dissolving in deuterated DMSO using a Bruker 500 MHz instrument. The X-ray diffraction data of the fibrous mats were analysed using the XRD-X'PERT PRO PANalytical instrument using the monochromatic CuKα radiation (λ = 1.5418 Å). The scans were performed in the 2θ range of 5–60° with a step size of 0.02° for every 1 s. Thermo gravimetric analysis (TGA) was carried out using a SDT 2600-TGA/DTA instrument at a heating rate of 10 °C min−1 from 37 °C to 800 °C under a constant nitrogen flow of 20 cm3 min−1. The Differential Scanning Calorimeter (DSC) measurements were performed using NETZSCH DSC 204 model in the temperature range between −100 °C to 150 °C and by using NETZSCH-Geratebu DSC 200PC model in the temperature range between 0 °C to 400 °C using nitrogen as the purge gas.DAPI staining
To analyse the level of cell attachment, DAPI (4′,6 diamidino-2-phenylindole) staining was performed as previously described.29 Briefly, the NIH 3T3 cells were seeded onto the PEUU and T-PEUU mats in 24 well plate. After 72 h incubation, mats were rinsed with phosphate buffered saline (PBS) and fixed with ice-cold 10% trichloroacetic acid. The mats were permeabilized with Triton-X (10% v/v) and stained with 1 μg ml−1 4′-6-diamidino-2-phenylindole for 3 minutes. To reduce the background, the stained mats were washed with PBS, 90% glycerol and observed under a fluorescence microscope (Labomed-Carl zeiss Lens with blue filter Olympus India). The morphology and adhesion of the cells seeded onto the fiber mats were observed by scanning electron microscopy (SEM).In vitro antibiotic release and antibacterial activity
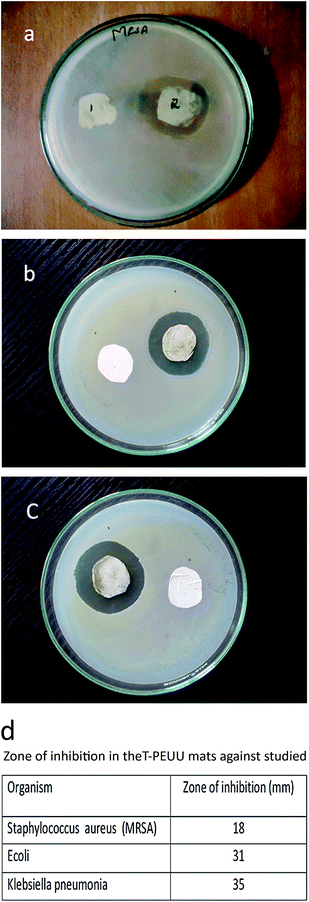
The ‘in vitro’ release of tigecycline from the T-PEUU mats was studied in phosphate buffered saline (PBS) at a pH of 7.4 and at a temperature of 37 °C for about 42 h. The concentration of the tigecycline in the aliquots was measured in the λmax of 245 and 378 nm respectively using a UV visible spectrophotometer. The antibacterial activity of the tigecycline loaded fibrous mats were analysed on Muller and Hinton agar using Kirby–Bauer disk diffusion method. The test pathogens were spread on the Muller Hinton agar test plates using the sterile swabs. The T-PEUU mats were then placed in the test pathogens at aseptic conditions. The test pathogens with the T-PEUU scaffolds were incubated for 24 h and the zone of inhibition was taken as the activity of the fibrous mats against the test organisms.Results and discussion
GPC analysis
The weight average molecular weight (Mw) and the number average molecular weight (Mn) of the synthesized PEUU were found to be about 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 127 and 48
127 and 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 156 respectively by the GPC analysis. The poly dispersity index was found to be 2.1, which shows that there was a broad molecular weight distribution in the synthesized PEUU polymer.
156 respectively by the GPC analysis. The poly dispersity index was found to be 2.1, which shows that there was a broad molecular weight distribution in the synthesized PEUU polymer.
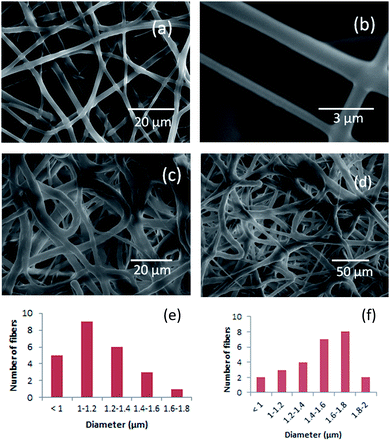
Characterization of electrospun PEUU and T-PEUU fibers
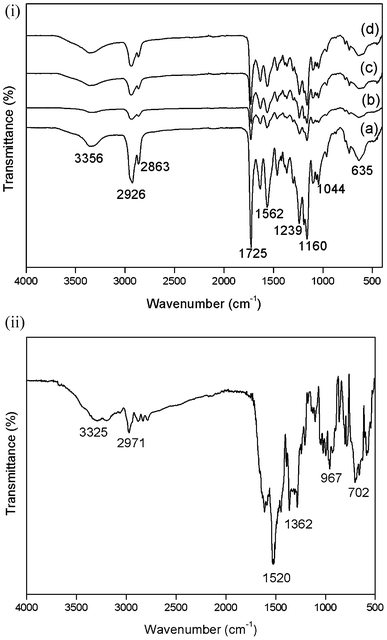
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the caprolactone and the urethane; the band at 1633 cm−1 indicates the C
O group of the caprolactone and the urethane; the band at 1633 cm−1 indicates the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of urea and the band at 1563 cm−1 represents the H-bonded amide.30 The molecular structure of the polymer and the electrospun mats remained the same, which confirmed that the conformation of the polyester urethane urea remained unaffected by the electrospinning process. The ATR-FTIR spectrum of the tigecycline (Fig. 2(e)) shows bands at 3325 and 3250 cm−1 due to the presence of O–H and N–H groups. The band at 2971 cm−1 represents the alkyl groups and the band for mono substituted benzene was present at 702 cm−1. The absorption bands of tigecycline and PEUU overlap each other due to the presence of similar functional groups.
O group of urea and the band at 1563 cm−1 represents the H-bonded amide.30 The molecular structure of the polymer and the electrospun mats remained the same, which confirmed that the conformation of the polyester urethane urea remained unaffected by the electrospinning process. The ATR-FTIR spectrum of the tigecycline (Fig. 2(e)) shows bands at 3325 and 3250 cm−1 due to the presence of O–H and N–H groups. The band at 2971 cm−1 represents the alkyl groups and the band for mono substituted benzene was present at 702 cm−1. The absorption bands of tigecycline and PEUU overlap each other due to the presence of similar functional groups.
1H NMR analysis
1H NMR spectrum of the electrospun scaffolds enables to analyse any structural changes that could have encountered during the electrospinning process. Fig. 3(a)–(d) shows the 1H NMR spectrum of the electrospun PEUU fibrous mat, T-PEUU, tigecycline hydrate and released tigecycline in PBS solution. 1H NMR (DMSO-d6, ppm) of electrospun PEUU mat: 0.7–1.7 (–CH3, –CH2, –CH protons in cyclohexyl ring) 2.2 (HC–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, carbonyl compounds), 3.9 (RCOO–CH esters) 5.8 (–NH, urea), 6.9–7.0 (–NHC
O, carbonyl compounds), 3.9 (RCOO–CH esters) 5.8 (–NH, urea), 6.9–7.0 (–NHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, urethane). The spectrum confirms the characteristic polyester urethane urea peaks in electrospun fibers. The 1H NMR signals (DMSO-d6, ppm) of tigecycline were present at 1.1 (–CH3), 2.2–3 (Ar–C–H) 8.3 (NH–C
O, urethane). The spectrum confirms the characteristic polyester urethane urea peaks in electrospun fibers. The 1H NMR signals (DMSO-d6, ppm) of tigecycline were present at 1.1 (–CH3), 2.2–3 (Ar–C–H) 8.3 (NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 9.1, 10.2 (Ar–OH). The prominent 1H NMR signals corresponding to both polyester urethane urea and tigecycline were observed in 1H NMR spectrum of T-PEUU. Tigecycline released in PBS (phosphate buffered saline solution) from PEUU fibers exhibits the peaks of tigecycline at 1.1, 2.4–3, 8.1 and 8.8 respectively. The results confirm the existence of tigecycline in the released solution.
O), 9.1, 10.2 (Ar–OH). The prominent 1H NMR signals corresponding to both polyester urethane urea and tigecycline were observed in 1H NMR spectrum of T-PEUU. Tigecycline released in PBS (phosphate buffered saline solution) from PEUU fibers exhibits the peaks of tigecycline at 1.1, 2.4–3, 8.1 and 8.8 respectively. The results confirm the existence of tigecycline in the released solution.
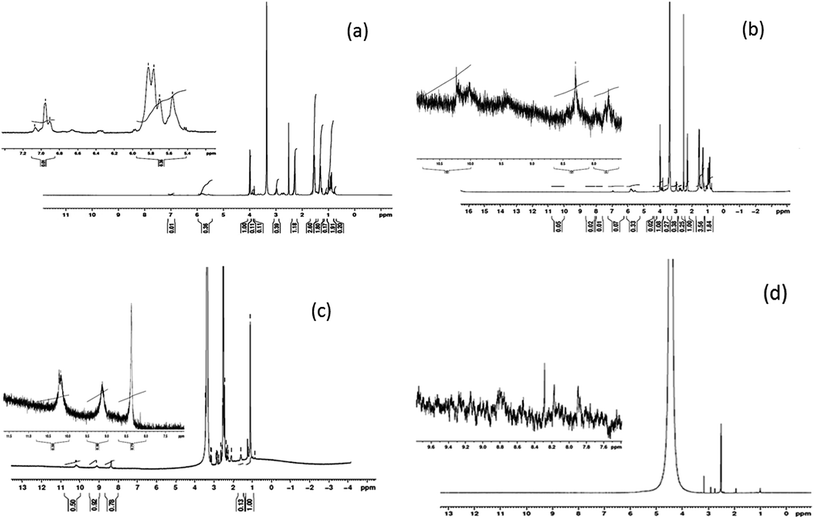 | ||
| Fig. 3 NMR spectrum of (a) electrospun PEUU fibers (b) electrospun T-PEUU fibers (c) tigecycline hydrate (d) released tigecycline from T-PEUU fibers in deuterated DMSO solvent. | ||
X-ray diffraction
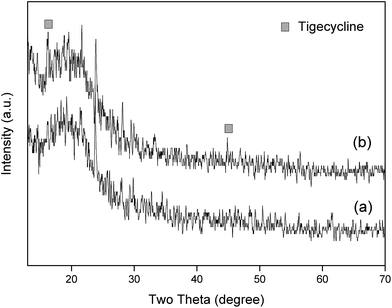
The X-ray diffraction (XRD) patterns of the electrospun PEUU scaffolds and the tigecycline loaded electrospun PEUU fibers are shown in the Fig. 4. The PEUU fibers and the T-PEUU fibers exhibited a characteristic polyurethane diffraction peak at 2θ = 20°. The 2θ values of tigecycline as reported31 were observed at 16° and 44° respectively in the T-PEUU fibers.Thermo gravimetric analysis
The relative thermal stability of the synthesized PEUU film, PEUU electrospun fibers and the tigecycline loaded PEUU fibers (T-PEUU) were studied by the TGA and DTA analysis. Fig. 5(a) shows the TGA and DTA curves of the synthesized PEUU film. The PEUU polymer showed two stage decompositions at the temperature of 318 and 430 °C respectively due to the decomposition of hard and soft segments in the polymer backbone. However, in the case of the electrospun PEUU fibers (Fig. 5(b)), DTA analysis of the electrospun PEUU showed decompositions at the temperature range of ∼300 and 436 °C respectively due to the decomposition of hard segments such as urea and urethane followed by the polycaprolactone soft segments respectively.32 Fig. 5(c) and (d) shows the TGA-DTA curve of the tigecycline loaded electrospun PEUU fibers. It was observed that the incorporation of the tigecycline increased the thermal stability of the hard segments in T-PEUU fibers.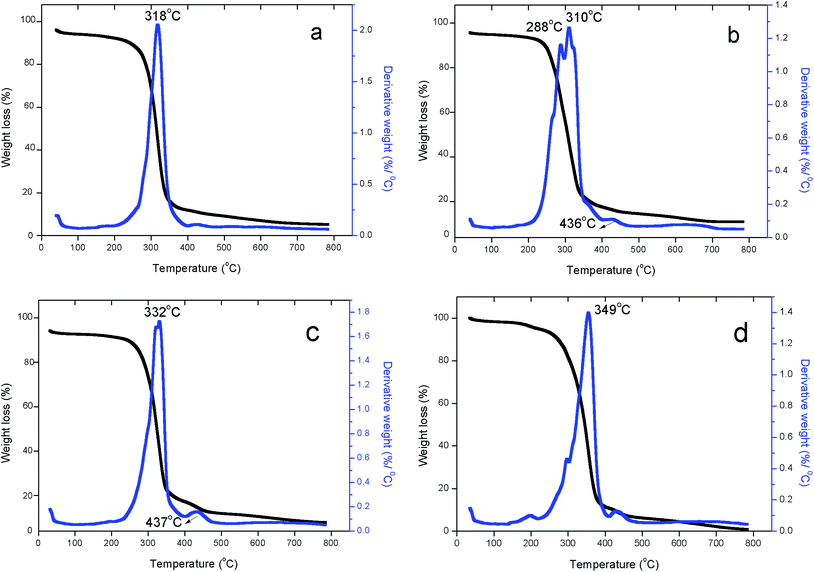 | ||
| Fig. 5 TGA-DTA thermograms of (a) PEUU raw polymer (b) electrospun PEUU fibers (c) T-PEUU fibers with 0.7 mg tigecycline (d) T-PEUU fibers with 1 mg tigecycline. | ||
Differential scanning calorimetry (DSC)
The DSC analysis was carried out to determine the glass transition, crystallization and the melting temperatures of the PEUU and T-PEUU fibers respectively. Fig. 6(a) and (b) shows the DSC curves of the PEUU and T-PEUU fibers respectively in the temperature ranges of (−100 to 150 °C) and (40 to 400 °C) respectively. The glass transition temperature (Tg) of the PEUU fibers was observed at −62 °C. Tigecycline is compatible with the polymer and acted as an antiplasticizer33 which led to an increase in the Tg value of T-PEUU fibers to −51 °C. In addition, the interactions between the N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of tigecycline with N–H and C
O groups of tigecycline with N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of PEUU fibers also contributed to an increase in the Tg value of T-PEUU fibers. Similarly, an increase in the melting temperatures of 53 °C for the PEUU fibers to 56 °C was also observed after the imbibition of the tigecycline into the PEUU fibers.
O groups of PEUU fibers also contributed to an increase in the Tg value of T-PEUU fibers. Similarly, an increase in the melting temperatures of 53 °C for the PEUU fibers to 56 °C was also observed after the imbibition of the tigecycline into the PEUU fibers.
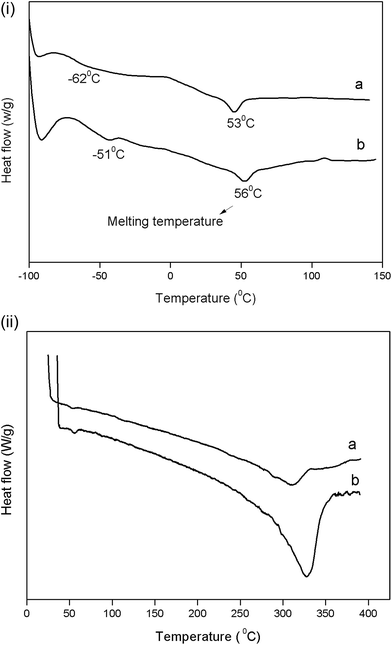 | ||
| Fig. 6 DSC thermograms of electrospun (i) (a) PEUU fibers (b) T-PEUU fibers in the temperature range of −100 to 150 °C (ii) (a) PEUU fibers (b) T-PEUU fibers in the temperature range of 0 to 400 °C. | ||
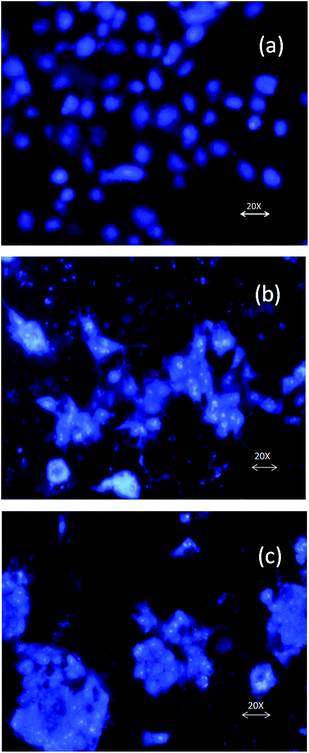
Biocompatibility of PEUU and T-PEUU fiber mats
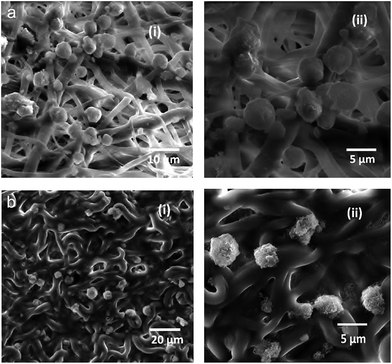
NIH 3T3 fibroblast cells cultured on the PEUU and T-PEUU fiber mats attached well to the microfiber surface. DAPI stained mats were examined under fluorescent microscope and the cell adhesion was confirmed by SEM analysis. Fig. 7(a)–(c) shows the fluorescent microscopic images (DAPI staining). The SEM images of NIH 3T3 seeded on PEUU and T-PEUU fiber mats at a 72 h period incubation are shown in Fig. 8(a) and (b).Antibacterial activity of the T-PEUU fibers and the in vitro release of the tigecycline
The methicillin resistant Staphylococcus aureus is one of the most common pathogen that affects the skin and soft tissues. Wounds are also vulnerable to the attack by the gram negative pathogen such as Escherichia coli and Klebsiella pneumonia. The antibacterial activities of the tigecycline loaded PEUU fibers (T-PEUU) were determined for methicillin resistant Staphylococcus aureus, Escherichia coli and Klebsiella pneumonia using the disk diffusion method. To assess the antibacterial activity, the release of the tigecycline from the fibrous mats were analysed for a 24 h period. T-PEUU fibrous mats at a tigecycline loading of 0.7 mg were found to exhibit good zone of inhibition against all the methicillin resistant organisms studied. The zones of the inhibition against the three strains are shown in Fig. 9(a)–(c). The ‘in vitro’ release of the 0.7 and 1 mg tigecycline from the T-PEUU scaffolds showed an initial burst release during the first five hours (i.e. ∼45 and 37%) followed by a sustained release of approximately 79 and 77% during the 42 h period (Fig. 10). The initial burst release could be due to the release of tigecycline present near the fiber surface. The sustained release at later stages could be attributed to the diffusion of the absorbed tigecycline into the PBS release medium. These results confirm the ease in the use of the electrospun T-PEUU fibrous mats to treat skin and soft tissue infections.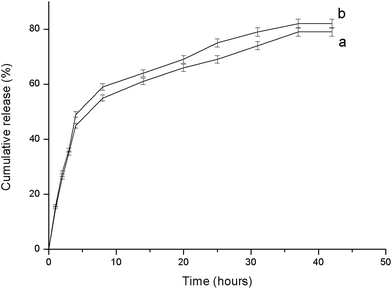 | ||
| Fig. 10 In vitro release study of tigecycline from T-PEUU scaffolds (a) ∼0.7 mg (b) 1 mg concentrations. | ||
Conclusion
In the present study, the synthesized PEUU was electrospun to produce a micro fibrous scaffold. Imbibition approach of loading tigecycline into the electrospun PEUU was carried out to assess the antibacterial activity of the prepared scaffold. The prepared T-PEUU fibers could be ideal to treat skin and soft tissue infections (SSTIs). The morphology and the diameters of the electrospun fibers were investigated using the SEM analysis. The electrospun PEUU and the T-PEUU scaffolds were characterized using the ATR-FTIR, 1H NMR, XRD, TGA, DTA and DSC analysis. The thermal analysis showed a slight increase in the thermal stability of the T-PEUU mats. The cell attachment studies confirmed the biocompatibility of the electrospun mats. The antibacterial activity of the prepared T-PEUU scaffolds were tested against the gram positive methicillin resistant Staphylococcus aureus and gram negative Escherichia coli and Klebsiella pneumonia which showed good zone of inhibition proving that the prepared fibrous scaffolds are suitable for biomedical applications.Acknowledgements
The authors gratefully acknowledge the financial assistance provided by University Grant Commision (UGC) (no. F 4-1/2006(BSR)/7-7/2007(BSR) dated 04.07.2011. The instrument facility provided under FIST-DST and DRS-UGC to the Department of Chemistry, Anna University, Chennai is acknowledged.References
- J. A. Nicholas, D. Antonio, Y. Hong and R. W. William, Adv. Mater., 2011, 23, 106 CrossRef PubMed.
- F. Dabirian, S. A. Hosseini Ravandi, A. R. Pishevar and R. A. Abuzade, J. Electrost., 2011, 69, 540 CrossRef PubMed.
- M. Sokolsky Papkov, K. Agashi, A. Olaye, K. Shakesheff and A. J. Domb, Adv. Drug Delivery Rev., 2007, 59, 187 CrossRef CAS PubMed.
- P. Zahedi, I. Rezaein, S. O. Ranaei-Siadat, S. H. Jafari and P. Supaphol, Polym. Adv. Technol., 2010, 21, 77 CAS.
- G. Alfred, K. Johanna, L. Thorsten, W. Torsten, N. Sylvia, G. Elke, B. Simone, W. Ralf, S. Matthias and K. Maike, PLoS One, 2014, 9, 90676 Search PubMed.
- P. C. Caracciolo, V. Thomas, Y. K. Vohra, F. Buffa and G. A. Abraham, J. Mater. Sci.: Mater. Med., 2009, 20, 2129 CrossRef CAS PubMed.
- A. Marcos-Fernandez, G. A. Abraham, J. L. Valentin and J. San Roma, Polymer, 2006, 47, 785 CrossRef CAS PubMed.
- L. P. Gabriel, C. A. C. Zavagliab, A. L. Jardinia, C. G. B. T. Diasc and R. M Filhoa, Chem. Eng. Trans., 2014, 38, 253 Search PubMed.
- M. Szycher, D. J. Dempsey and J. L. Rolfe, US pat., Number 32,991, 1989.
- S. Gopalakrishnan and T. L. Fernando, J. Chem. Pharm. Res., 2011, 3(2), 848–862 CAS.
- S. Sarkar, A. Chourasia, S. Maji, S. Sadhukhan, S. Kumar and B. Adhikari, Bull. Mater. Sci., 2006, 29, 475 CrossRef CAS.
- S. Akita, K. Akino, T. Imaizumi, K. Tanaka, K. Anraku, H. Yano and A. Hirano, Burns, 2006, 32, 447–451 CrossRef PubMed.
- H. J. Yoo and H. D. Kim, J. Biomed. Mater. Res., Part B, 2008, 85, 326–333 CrossRef PubMed.
- W. L. J. Hinrichs, E. J. C. M. P. Lommen, C. R. H. Wildevuur and J. Feijen, J. Appl. Biomater., 1992, 3, 287–303 CrossRef CAS PubMed.
- L. I. F. Moura, A. M. A. Dias, E. Carvalho and H. C. de Sousa, Acta Biomater., 2013, 9, 7093–7114 CrossRef CAS PubMed.
- M. S. Khil, D.-I. Cha, H. Y. Kim, I. S. Kim and N. Bhattarai, J. Biomed. Mater. Res., Part B, 2003, 67, 675 CrossRef PubMed.
- C. B. Washington, S.-H. Ye, H. Sakaguchi, A. J. Shukla, W. R. Wagner and E. Tzeng, J. Am. Coll Surg, 2014, 219, S160 CrossRef PubMed.
- C. Hacker, Z. Karahaliloglu, G. Seide, E. B. Denkbas and T. Gries, J. Appl. Polym. Sci., 2014, 131, 40132 CrossRef.
- S. E. Kim, D. N Heo, J. B Lee, J. R Kim, S. H Park, S. H Jeon and I. K Kwon, Biomed. Mater., 2009, 4, 044106 CrossRef PubMed.
- A. R. Unnithan, N. A. Barakat, P. B. Pichiah, G. Gnanasekaran, R. Nirmala, Y. S. Cha, C. H. Jung, M. EI-Newehy and H. Y. Kim, Carbohydr. Polym., 2012, 90, 1786–1793 CrossRef CAS PubMed.
- B. I. Eisenstein, Clin. Microbiol. Infect., 2008, 14, 17–25 CrossRef PubMed.
- Y. Cai, R. Wang, B. Liang, N. Bai and Y. Liu, Antimicrob. Agents Chemother., 2011, 55, 1162–1172 CrossRef CAS PubMed.
- J. I. Merlino and M. A. Malangoni, Cleve Clin J Med, 2007, 74, 21 CrossRef.
- D. G. Nickie, Pharmacology notes, Proc (Bayl Univ Med Cent), 2006, vol. 19, p. 155 Search PubMed.
- M. Zamani, M. P. Prabhakaran and S. Ramakrishna, International journal of Nanomedicine, 2013, 8, 2997 Search PubMed.
- M. Sabitha and S. Rajiv, Polym. Eng. Sci., 2014 DOI:10.1002/pen23917.
- D. Liang, S. B. Hsiao and B. Chu, Adv. Drug Delivery Rev., 2007, 59, 1392 CrossRef CAS PubMed.
- J. Guan, K. L. Fujimoto, M. S. Sacks and R. W. William, Biomaterials, 2005, 26, 3961 CrossRef CAS PubMed.
- F. Sandra, M. Matsuda, H. Yoshida and M. Hirata, Carcinogenesis, 2002, 23, 2031 CrossRef PubMed.
- K. K. Jena, D. K. Chattopadhyay and K. V. S. N. Raju, Eur. Polym. J., 2007, 43, 1825 CrossRef CAS PubMed.
- N. L. Ignjatovic, P. Ninkov, R. Sabetrasekh and D. P. Uskokovic, J. Mater. Sci.: Mater. Med., 2010, 21, 231 CrossRef CAS PubMed.
- L. Haoxiang, S. Peiyu, Z. Zhen, Y. Xiong, W. Xinling and C. Feng-Chih, Polym. Degrad. Stab., 2012, 97, 661 CrossRef PubMed.
- E. Snejdrova and M. Dittrich, Pharmaceutically Used Plasticizers, www.intechopen.com, accessed 21 March 2012 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |