High catalytic activity of a PbS counter electrode prepared via chemical bath deposition for quantum dots-sensitized solar cells
Yanli Chena,
Xiaolin Zhanga,
Qiang Taoa,
Wuyou Fu*a,
Haibin Yanga,
Shi Sua,
Yannan Muab,
Liying Zhoua and
Minghui Lia
aState Key Laboratory of Superhard Materials, Jilin University, Changchun 130012, PR China. E-mail: fuwy@jlu.edu.cn
bDepartment of Physics and Chemistry, Heihe University, Heihe 164300, PR China
First published on 25th November 2014
Abstract
A PbS counter electrode (CE) has been fabricated by a chemical bath deposition method, and can function as a counter electrode with high catalytic activity for quantum dots-sensitized solar cells (QDSSCs). The PbS nanoparticles can act as an excellent electrical tunnel for fast electron transport from an external circuit to the CE. Electrochemical impedance spectroscopy reveals a low charge transfer resistance (Rct1) between polysulfide and PbS, the optimized PbS CE shows an Rct1 as low as 15.42 Ω cm2. The current density–voltage curves of the QDSSCs were investigated under AM 1.5 light at 100 mW cm−2. CdS quantum dots-sensitized solar cells with the PbS CE achieved a power energy conversion efficiency of 2.91% and showed no obvious degradation of current density over 72 h under ambient conditions.
1. Introduction
Dye-sensitized solar cells (DSSCs) have been investigated extensively during the past two decades, since O’Regan and Grätzel first reported them in 1991.1 As an alternative, quantum dots (QDs)-sensitized solar cells (QDSSCs) have attracted much attention recently, because of the potential of semiconductor QDs with properties such as a high extinction coefficient, tunable band gap, large intrinsic dipole moment, and low processing cost as compared to organic dyes.2–6 The fabrication of QDSSCs is cost effective and simple. Normally, they are composed of a wide band gap semiconductor photoelectrode, sensitizer, counter electrode and redox electrolyte. The role of the semiconductor electrode is to acquire photo-injected electrons from the sensitizer and provide a good electron transport bridge between the sensitizer and external circuit. Over the past few years, there have been quite a few studies on the improvement and modification of the semiconductor photoelectrode, such as the design and optimization of methods for the deposition of QDs on the semiconductor photoelectrode,7–10 optimization of the semiconductor photoelectrode structure,11–13 and employing new kinds of quantum dots sensitizers.14–16 An impressive efficiency of up to 5.4% has recently been achieved with Mn-doped CdS/CdSe QDs-sensitized photoanodes.17Although much effort has been devoted to the development of QDSSCs, their photovoltaic efficiency is still relatively low. One major challenge in this field is how to select the counter electrode (CE) and the electrolyte; both are equally important to improve the performance of QDSSCs. As we know, DSSCs based on an organic electrolyte consisting of the I−/I3− redox couple show the highest performance, but the I−/I3− redox electrolyte cannot be suitable for QDSSCs, because it causes corrosion and photodegradation of QDs.18 Consequently, polysulfide redox couples (S2−/Sn2−) have often been used in QDSSCs to ensure the stability of the photoanode.19–22 The Pt electrode is the most widespread CE in DSSCs, showing a low charge transfer resistance (Rct), with Pt always being the catalytic electrode for the organic I−/I3− redox electrolyte. However, in conjunction with aqueous polysulfide electrolytes, Pt is not a suitable catalytic electrode, because Pt can generate considerable overpotentials for the regeneration of the polysulfide electrolyte and introduce a serial resistance, which is reflected by a reduction of the total conversion efficiency of the solar cell. The efficient charge transfer between sulfide sensitizers and polysulfide redox couples generates a large short-circuit photocurrent (Jsc) in QDSSCs.23–25 However, the large charge transfer resistance at the counter electrode results in a low fill factor (FF), and the small difference between the semiconductor photoelectrode Fermi level and the S2−/Sn2− redox level confines the open-circuit photovoltage (Voc). A promising strategy to increase the Voc value without sacrificing the high Jsc is to use a narrow band gap metal chalcogenide as the CE to provide an auxiliary tandem effect.26 Therefore, in order to overcome the limitations of the CE and improve the cell performance, several kinds of catalytic electrode materials for polysulfide electrolyte have been investigated to replace the traditional Pt electrode, such as CoS, Cu2S, CuS, NiS, and PbS.27–35 Among all these CE materials, PbS shows promising stability as well as good catalytic activity. PbS has been considered as the perfect electrode candidate due to its low cost, highly catalytic effect towards the polysulfide reaction, and superior chemical and electrochemical stabilities. Zaban et al. prepared a PbS CE on Pb foil; the Rct was 130 Ω cm2 and the CdS/CdSe QDSSCs based on this CE obtained an efficiency of 3.01%.31 Meng et al. prepared a PbS/carbon black (CB) composite CE for highly efficient CdS/CdSe QDSSCs, for which the best efficiency so far of 3.91% was obtained.36 Zhang et al. used PbS as the CE for a CdSe QDs-sensitized ZnO (SnO2) thin film; the light to electric power conversion efficiency (η) was greatly increased from 1.21% to 1.75%.37
In this paper, we report a chemical bath deposition (CBD) method for the preparation of a highly catalytic PbS CE on FTO substrates for highly efficient CdS quantum dots (QDs)-sensitized actinomorphic hexagonal columnar ZnO (CdS QDs/AHC-ZnO) solar cells with ZnS as a passivation layer. X-ray diffraction spectroscopy, field-emission scanning electron microscopy, high-resolution transmission electron microscopy and selected area electron diffraction (SAED) patterns were used to characterize the structural properties and morphology of the PbS CE. Electrochemical impedance spectroscopy and cyclic voltammetry were employed to analyze the compatibility and catalytic mechanism between counter electrodes and the polysulfide electrolyte, and the results show that the prepared PbS CE has better catalytic activity and electrochemical stability for polysulfide electrolyte. It can greatly increase the photoelectric performance of QDSSCs. The photoelectric performance of QDSSCs can be reflected by current density–voltage curve measurements under illumination, and the solar cell performance of a Pt CE and Cu2S CE were also obtained to compare with the PbS CE. Our results demonstrate that the PbS CE shows outstanding electrocatalytic activity as well as unsurpassed stability in polysulfide electrolyte.
2. Experimental section
Preparation of PbS counter electrode
In a typical synthesis, FTO substrates were ultrasonically cleaned sequentially in acetone, isopropanol, and ethanol (15 min in each), and were finally dried with nitrogen gas. The PbS counter electrode was prepared by a CBD method. Typically, 0.05 M lead acetate and 0.05 M thiourea solutions with ethylenediamine as the solvent was prepared separately, mixed, and then the FTO was vertically immersed into the deposition solution at 60 °C for 3 h. After completion of the deposition process, the coated substrates were cleaned with water and ethanol in an ultrasound bath for 5 min, and then dried under a high-purity nitrogen flow.For the purpose of comparison, in this study we prepared PbS CEs with different growth times. These were kept in the deposition solution at 60 °C for 1 h and 5 h, and are denoted PbS1 and PbS2, respectively.
Preparation of photoanodes and QDSSCs
The fabrication of actinomorphic hexagonal columnar ZnO (AHC-ZnO) was based on our previous study.38 100 mL of 0.1 M aqueous zinc nitrate hexahydrate solution was mixed with 100 mL of 0.1 M aqueous hexamethylenetetramine solution and kept under mild magnetic stirring for 10 min. Then, the mixture was transferred into a 500 mL beaker containing the cleaned FTO substrate. The system was heated at 90 °C in an electric constant temperature water bath pot for 24 h with refluxing. After synthesis, the FTO substrates were taken out and thoroughly washed with DI water/ethanol, and then dried under a high-purity nitrogen flow. Finally, the samples were annealed at 500 °C for 2 h in air.The successive ionic layer adsorption and reaction (SILAR) technique was employed to fabricate CdS QDs on the photoanodes. For the CdS sensitizer, the photoelectrodes were immersed in a 0.5 M cadmium nitrate tetrahydrate solution in ethanol for 5 min, then rinsed with ethanol to remove the excess Cd2+. The photoelectrodes were dried under a high-purity nitrogen flow for 2 min. Subsequently, the dried photoelectrodes were dipped into 0.5 M sodium sulfide nonahydrate in a mixture of methanol and deionized water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for 5 min. The photoelectrodes were then rinsed with methanol and dried again with N2. All of the above was considered as one SILAR cycle. The incorporated amount of sensitizer could be increased by repeating the assembly cycle. Finally, ZnS passivation layer was coated on the photoanodes about five cycles by immersing alternately into 0.1 M zinc acetate dihydrate and 0.1 M sodium sulfide nonahydrate aqueous solutions each for 1 min.
1, v/v) for 5 min. The photoelectrodes were then rinsed with methanol and dried again with N2. All of the above was considered as one SILAR cycle. The incorporated amount of sensitizer could be increased by repeating the assembly cycle. Finally, ZnS passivation layer was coated on the photoanodes about five cycles by immersing alternately into 0.1 M zinc acetate dihydrate and 0.1 M sodium sulfide nonahydrate aqueous solutions each for 1 min.
The samples were sealed in sandwich structures with a 60 μm spacer by using PbS as the counter electrode. The polysulfide electrolyte composed of a 1 M Na2S and 1 M S solution in Milli-Q ultrapure water was injected between the two electrodes. A mask with a window of 0.25 cm2 was clipped onto the AHC-ZnO side to define the active area of the cell.
Characterization
The structural properties and morphologies of the samples were characterized by X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), high-resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) patterns. XRD patterns were obtained using a Rigaku D/max-2500 X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å). FESEM was carried out using a JEOL JEM-6700F microscope operated at 8 KV. High-resolution transmission electron microscopy (HRTEM) images and selected area electron diffraction (SAED) patterns were obtained on a JEM-2100F microscope with an accelerated voltage of 200 KV. Electrochemical impedance spectroscopy (EIS) measurements were performed with a CHI650D electrochemical workstation in the frequency range from 0.02 to 105 Hz. The magnitude of the alternative signal was 10 mV. Cyclic voltammetry (CV) was performed using a CHI601D electrochemical workstation with the CE in polysulfide electrolyte at a scan rate of 50 mV s−1 and with a saturated calomel electrode (SCE) as the reference electrode. Current density–voltage curve measurements were performed with a Keithley model 2400 Source Meter and a 500 W Xe lamp (Spectra Physics) with a monochromator to simulate sunlight. A laser power meter (BG26M92C, Midwest Group) was used to revise the light intensity, such that it was as effective as an AM 1.5 light at 100 mW cm−2. The incident photon-to-current conversion efficiency (IPCE) was measured with an action spectrum measurement setup (PEC-S20, Peccell Ltd.).3. Results and discussion
The PbS counter electrode (PbS CE) prepared by the CBD process was characterized by XRD (Fig. 1). After subtracting the diffraction peaks of the FTO substrate, three diffraction peaks were observed in the sample centered at 2θ angles of 26.05°, 30.18° and 43.21°, corresponding to PbS (111), (200) and (220) planes, respectively. These peaks for the corresponding crystal planes match very well to cubic phase PbS (JCPDS no. 78-1898).Fig. 2 shows the morphological and structural characterization of PbS CE. The cross section of the PbS CE (Fig. 2a) reveals that the PbS layer thickness is about 200 nm. Most of the PbS nanoparticles synthesized are around 50–100 nm (Fig. 2b), producing long-range uniformity in the PbS film. To further obtain structural information for the PbS nanoparticles, a typical HRTEM image (Fig. 2c) and SAED pattern (Fig. 2d) were also recorded for a single nanoparticle. The (200) lattice plane of cubic phase PbS, with a lattice spacing of about 2.96 Å, can be clearly identified in the lattice-resolved HRTEM image (Fig. 2c). The [001] SAED (Fig. 2d) demonstrates an apparent cubic phase, conforming with the results from XRD and HRTEM.
Electrochemical impedance spectroscopy (EIS) measurements, which are widely used for characterizing sensitized solar cells,39 have been performed with a standard three-electrode configuration using a saturated calomel electrode (SCE) as the reference electrode to derive the charge transfer resistance between the CE and the polysulfide electrolyte. Symmetric cells40 composed of a working electrode and CE placed face to face were fabricated for EIS measurements. The area of each CE was 0.5 cm2. An aqueous solution with 1 M concentrations of Na2S and S was used as the electrolyte. The equivalent circuit of the system is depicted in Fig. 3a. This includes the series resistance (Rs) at a high frequency of around 100 kHz and in the frequency range between 100 Hz and 10 kHz; the impedance associated with the electron transfer at the CE/electrolyte interface, which consists of the charge transfer resistance (Rct1) and the double layer capacitance (CPE1, constant phase element);41 and the Nernst diffusion, consisting of the Nernst diffusion impedance (Rct2) of the S2−/Sx2− redox couple of the electrolyte and the capacitance (CPE2) in the low-frequency region.
From Fig. 3b and c, fitting the impedance data with the equivalent circuit model produced Rct1 values of 593.28 and 3.5 Ω cm2 for the Pt and Cu2S electrodes, respectively. The Pt electrode had a strong resistance to interaction with the polysulfide electrolyte, suggesting that its catalytic activity is very poor in polysulfide electrolyte, whereas the Cu2S electrode exhibited a much lower Rct1 for interaction with the polysulfide electrolyte. The EIS measurements of the electrodes produced Rct1 values of 15.42, 23.99 and 29.74 Ω cm2 for the PbS, PbS1 and PbS2 electrodes, respectively. The resistance of PbS1 is higher than the PbS CE; this may be due to the fact that the nanoparticles in PbS1 were not fully grown, and the film could not be connected well. The high Rct1 of the PbS2 electrode suggests that extending the reaction time is not conducive to the charge transfer of the PbS CEs. This may be due to the fact that as the PbS nanoparticles grow, the poor connections between the PbS nanoparticles create obstacles to the transportation of electrons flowing from the external circuit to the PbS/electrolyte interface. This means that the catalytic sites on the PbS nanoparticles may not be fully utilized, thus resulting in a higher overall Rct1 value.
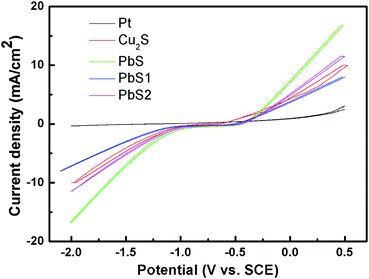
In order to further investigate the reaction kinetics and electrocatalytic activities of the CEs in polysulfide electrolyte, cyclic voltammograms (CV) were obtained with a standard three-electrode electrochemical system.42 The three-electrode system was composed of a working electrode, Pt net counter electrode, and SCE reference electrode. The corresponding CV curves are shown in Fig. 4. In QDSSCs, photo-excited electrons from the QDs are injected into the conducting band of AHC-ZnO. The oxidized QDs are reduced back by S2− ions in the electrolyte. The simultaneous presence of sulfur with S2− leads to the formation of polysulfide (Sx2−, x = 2–5), and the produced Sx2− ions are then reduced at the CE;43 the redox reactions at the photoanode and at the CE can be represented by eqn (1) and (2), respectively:27
| S2− − 2e− → S; S + S2− → Sx2− (x = 2–5) | (1) |
| Sx2− + 2e− → Sx−12− + S2− (x = 2–5) | (2) |
The PbS CE shows a much higher current density relative to that of Pt, Cu2S and other PbS CEs. This is because the reduction rate of Sx2− is higher on the PbS CE compared to other CEs, and hence it tends to have a higher current density.44–46 This result also reveals that the PbS CE has a better electrocatalytic ability in polysulfide electrolyte. The EIS and CV results confirm that the catalytic activity of the PbS CE towards the polysulfide redox shuttle is superior to that of Pt, Cu2S and other PbS CEs, in accordance with its excellent photovoltaic performance.
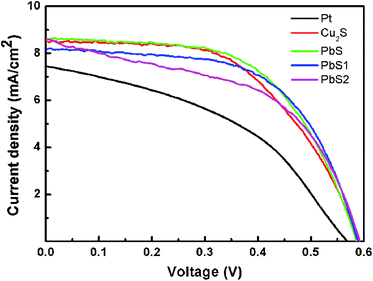
We further investigated the photoelectric performance of the PbS CE in QDSSCs. The current density–voltage curve characteristics of QDSSCs employing different CEs along with ZnS/CdS/AHC-ZnO photoanodes were evaluated. An aqueous solution with 1 M concentrations of Na2S and S was used as the polysulfide electrolyte. The current density–voltage curves of these cells measured under AM 1.5 light at 100 mW cm−2 are shown in Fig. 5. Detailed data are presented in Table 1. The QDSSCs with a Pt CE show quite poor performance, with Jsc, Voc and FF values of 7.44 mA cm−2, 0.57 V and 43%, respectively, resulting in a low power energy conversion efficiency (η) of 1.81%. This low η value was due to the low catalytic activity of the Pt CE in the polysulfide electrolyte, which leads to a low FF value. Compared with the Pt electrode, Cu2S had significantly improved Jsc and FF values of 8.50 mA cm−2 and 55%, respectively, resulting in a η value of 2.75%. This is primarily because of the relatively low Rct1. The QDSSCs assembled with the PbS CE exhibited better performance (η = 2.91%) than the cells with other CEs, due to the higher Jsc, Voc and FF values, even though PbS had a larger Rct1 than Cu2S. The fact that the performance of the PbS electrode is better than Cu2S is attributed to the electrocatalytic ability of Cu2S being weaker than PbS, such that the reduction rate with Cu2S is low for the polysulfide electrolyte.
| Counter electrode | Jsc (mA cm−2) | Voc (V) | FF | η (%) |
|---|---|---|---|---|
| Pt | 7.44 | 0.57 | 0.43 | 1.81 |
| Cu2S | 8.50 | 0.59 | 0.55 | 2.75 |
| PbS | 8.59 | 0.59 | 0.58 | 2.91 |
| PbS1 | 8.15 | 0.59 | 0.60 | 2.86 |
| PbS2 | 8.61 | 0.59 | 0.51 | 2.61 |
Incident photon-to-current conversion efficiency (IPCE) is defined as the number of electrons in the external circuit produced at a given wavelength divided by the number of incident photons.47 Fig. 6 shows the IPCE of the QDSSCs with Pt, Cu2S, and different kinds of PbS CEs. The IPCE curves cover the spectrum range from 350 to 520 nm. The QDSSCs based on the PbS CE shows a higher IPCE value than that of the cells with other CEs, which is in accord with the results of the current density–voltage measurements, demonstrating the excellent performance of PbS as a CE for polysulfide electrolyte.
Fig. 7 shows the long-term stability of the PbS CE. The test was carried out to observe whether the PbS CE is stable in QDSSCs.
The sealed QDSSCs with different CEs were kept under ambient conditions for 72 h, with the current density measured under AM 1.5 light at 100 mW cm−2 illumination every 24 h. The QDSSCs with the PbS CE showed no obvious degradation of current density over such a long time. The results clearly demonstrate the much better stability of the PbS CE compared with the CE based on Cu2S, for which the current density fell to around 86% of its initial value after 3 days.
4. Conclusions
This study reports the synthesis of a PbS counter electrode via chemical bath deposition for high performance QDSSCs. Pt, Cu2S and PbS CEs with various growth times have been systematically studied by EIS and current density–voltage measurements. When the growth time was 3 h, QDSSCs with the PbS CE achieved an optimized efficiency of up to 2.91%, and the PbS CE exhibited a lower Rct1 value of 15.42 Ω cm2. The stability test for QDSSCs with the PbS CE indicated that there is no obvious degradation of current density after 72 h under ambient conditions. This proves that the PbS CE is a highly stable CE for QDSSCs.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 51272086) and Science and Technology Development Program of Jilin Province (20100417).Notes and references
- B. O’Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef.
- P. V. Kamat, J. Phys. Chem. C, 2008, 112, 18737 CAS.
- Z. S. Yang, C. Y. Chen, P. Roy and H. T. Chang, Chem. Commun., 2011, 47, 9561 RSC.
- P. Chen, L. Gu and X. B. Cao, CrystEngComm, 2010, 12, 3950 RSC.
- L. M. Peter, K. G. U. Wijayantha, D. J. Riley and J. P. Waggett, J. Phys. Chem. B, 2003, 107, 8378 CrossRef CAS.
- S. Buhbut, S. Itzhakov, E. Tauber, M. Shalom, I. Hod, T. Geiger, Y. Garini, D. Oron and A. Zaban, ACS Nano, 2010, 4, 1293 CrossRef CAS PubMed.
- J. B. Yin and J. H. Jia, CrystEngComm, 2014, 16, 2795 RSC.
- J. W. Ma, S. Su, W. Y. Fu, H. B. Yang, X. M. Zhou, H. Z. Yao, Y. L. Chen, L. H. Yang, M. L. Sun, Y. N. Mu and P. Lv, CrystEngComm, 2014, 16, 2910 RSC.
- S. C. Lin, Y. L. Lee, C. H. Chang, Y. J. Shen and Y. M. Yang, Appl. Phys. Lett., 2007, 90, 143517 CrossRef PubMed.
- H. J. Lee, M. K. Wang, P. Chen, D. R. Gamelin, S. M. Zakeeruddin, M. Grätzel and M. K. Nazeeruddin, Nano Lett., 2009, 9, 4221 CrossRef CAS PubMed.
- W. Lee, S. H. Kang, S. K. Min, Y. E. Sung and S. H. Han, Electrochem. Commun., 2008, 10, 1579 CrossRef CAS PubMed.
- E. Y. Guo, L. W. Yin and L. Y. Zhang, CrystEngComm, 2014, 16, 3403 RSC.
- J. F. Chao, Z. Xie, X. B. Duan, Y. Dong, Z. R. Wang, J. Xu, B. Liang, B. Shan, J. H. Ye, D. Chen and G. Z. Shen, CrystEngComm, 2012, 14, 3163 RSC.
- H. Lee, H. C. Leventis, S. J. Moon, P. Chen, S. Ito, S. A. Haque, T. Torres, F. Nüesch, T. Geiger, S. M. Zakeeruddin, M. Grätzel and M. K. Nazeeruddin, Adv. Funct. Mater., 2009, 19, 2735 CrossRef CAS.
- T. L. Li, Y. L. Lee and H. Teng, Energy Environ. Sci., 2012, 5, 5315 CAS.
- K. P. Kadlag, P. Patil, M. J. Rao, S. Datta and A. Nag, CrystEngComm, 2014, 16, 3605 RSC.
- P. K. Santra and P. V. Kamat, J. Am. Chem. Soc., 2012, 134, 2508 CrossRef CAS PubMed.
- M. Shalom, S. Dor, S. Rühle, L. Grinis and A. Zaban, J. Phys. Chem. C, 2009, 113, 3895 CAS.
- G. Syrrokostas, A. Siokou, G. Leftheriotis and P. Yianoulis, Sol. Energy Mater. Sol. Cells, 2012, 103, 119 CrossRef CAS PubMed.
- J. J. Tian, R. Gao, Q. F. Zhang, S. G. Zhang, Y. W. Li, J. Lan, X. H. Qu and G. Z. Cao, J. Phys. Chem. C, 2012, 116, 18655 CAS.
- N. Guijarro, E. Guillén, T. L. Villarreal and R. Gómez, Phys. Chem. Chem. Phys., 2014, 16, 9115 RSC.
- R. Zhou, Q. F. Zhang, E. Uchaker, J. Lan, M. Yin and G. Z. Cao, J. Mater. Chem. A, 2014, 2, 2517 CAS.
- G. Hodes, J. Manassen and D. Cahen, J. Electrochem. Soc., 1980, 127, 544 CrossRef CAS PubMed.
- B. Miller and A. Heller, Nature, 1976, 262, 680 CrossRef CAS.
- J. Xu, X. Yang, Q. D. Yang, T. L. Wong, S. T. Lee, W. J. Zhang and C. S. Lee, J. Mater. Chem., 2012, 22, 13374 RSC.
- C. Y. Lin, C. Y. Teng, T. L. Li, Y. L. Lee and H. Teng, J. Mater. Chem. A, 2013, 1, 1155 CAS.
- Z. Yang, C. Y. Chen, C. W. Liu and H. T. Chang, Chem. Commun., 2010, 46, 5485 RSC.
- C. Shen, L. D. Sun, Z. Y. Koh and Q. Wang, J. Mater. Chem. A, 2014, 2, 2807 CAS.
- Z. Yang, C. Y. Chen, C. W. Liu, C. L. Li and H. T. Chang, Adv. Energy Mater., 2011, 1, 259 CrossRef CAS.
- H. N. Chen, L. Q. Zhu, H. C. Liu and W. P. Li, J. Phys. Chem. C, 2013, 117, 3739 CAS.
- Z. Tachan, M. Shalom, I. Hod, S. Rühle, S. Tirosh and A. Zaban, J. Phys. Chem. C, 2011, 115, 6162 CAS.
- J. N. Freitas, A. S. Gonçalves and A. F. Nogueira, Nanoscale, 2014, 6, 6371 RSC.
- D. H. Youn, M. Seol, J. Y. Kim, J. W. Jang, Y. Choi, K. Yong and J. S. Lee, ChemSusChem, 2013, 6, 261 CrossRef CAS PubMed.
- M. Seol, D. H. Youn, J. Y. Kim, J. W. Jang, M. Choi, J. S. Lee and K. Yong, Adv. Energy Mater., 2014, 4 DOI:10.1002/aenm.201300775.
- T. H. Thanh, D. H. Thanh and V. Q. Lam, Adv. OptoElectron., 2014, 2014, 397681 Search PubMed.
- Y. Y. Yang, L. F. Zhu, H. C. Sun, X. M. Huang, Y. H. Luo, D. M. Li and Q. B. Meng, ACS Appl. Mater. Interfaces, 2012, 4, 6162 CAS.
- J. B. Zhang, F. Y. Zhao, G. S. Tang and Y. Lin, J. Solid State Electrochem., 2013, 17, 2909 CrossRef CAS PubMed.
- Y. L. Chen, Q. Tao, W. Y. Fu, H. B. Yang, X. M. Zhou, Y. Y. Zhang, S. Su, P. Wang and M. H. Li, Electrochim. Acta, 2014, 118, 176 CrossRef CAS PubMed.
- L. Y. Han, N. Koide, Y. Chiba, A. Islam, R. Komiya, N. Fuke, A. Fukui and R. Yamanaka, Appl. Phys. Lett., 2005, 86, 213501 CrossRef PubMed.
- I. M. Seró, S. Giménez, F. F. Santiago, R. Gómez, Q. Shen, T. Toyoda and J. Bisquert, Acc. Chem. Res., 2009, 42, 1848 CrossRef PubMed.
- A. Haucha and A. Georg, Electrochim. Acta, 2001, 46, 3457 CrossRef.
- L. Li, X. C. Yang, J. Z. Zhao, J. J. Gao, A. Hagfeldt and L. C. Sun, J. Mater. Chem., 2011, 21, 5573 RSC.
- Y. L. Lee and C. H. Chang, J. Power Sources, 2008, 185, 584 CrossRef CAS PubMed.
- J. G. Radich, R. Dwyer and P. V. Kamat, J. Phys. Chem. Lett., 2011, 2, 2453 CrossRef CAS.
- C. J. Raj, K. Prabakar, A. D. Savariraj and H. J. Kim, Electrochim. Acta, 2013, 103, 231 CrossRef PubMed.
- X. H. Song, M. Q. Wang, J. P. Deng, Y. Y. Ju, T. Y. Xing, J. J. Ding, Z. Yang and J. Y. Shao, J. Power Sources, 2014, 269, 661 CrossRef CAS PubMed.
- J. R. Mann, M. K. Gannon, T. C. Fitzgibbons, M. R. Detty and D. F. Watson, J. Phys. Chem. C, 2008, 112, 13057 CAS.
| This journal is © The Royal Society of Chemistry 2015 |