Copper-catalyzed aerobic synthesis of bisaryl ketones from alkynes via the cleavage of C–C triple bonds†
Lijun Gu* and
Hongtao Zhang
Key Laboratory of Chemistry in Ethnic Medicinal Resources, State Ethnic Affairs Commission & Ministry of Education, Yunnan Minzu University, Kunming, Yunnan 650500, China. E-mail: gulijun2005@126.com
First published on 1st December 2014
Abstract
A novel copper-catalyzed aerobic synthesis of bisaryl ketones from 1,2-diarylalkynes via the cleavage of C–C triple bonds is reported. This reaction is a new transformation of 1,2-diarylalkynes into bisaryl ketones.
The alkyne functional group is found in numerous natural products, bioactive compounds and organic materials as well as in versatile intermediate compounds in organic synthesis.1–6 The transformation of alkynes is a fundamental method that has been widely used in organic synthesis. The classical elaborations of alkynes include hydration reactions,7 addition reactions,8 Wacker-type oxidation,9 cyclization reactions and coupling reactions.10–12 The catalytic cleavage of C–C triple bonds to produce carboxylic acids and new alkynes has also been reported.13 Shen et al.14 recently made the remarkable observation that alkynes react with TMSN3 in the presence of Ag2CO3 as a catalyst to form nitriles. The same group of workers has reported the cleavage of the aryl–alkyne bond of alkynes to form carboxamides.15 As a result of the significance and wide application of such chemical reactions in organic synthesis, new types of alkyne transformations are now being actively explored.
Saturated carbonyl compounds, e.g. ketones, are essential synthetic elements in organic chemistry and can be transformed into a large variety of functionalized organic molecules with applications in several different fields, including pharmaceutical chemistry and materials science.16,17 Their importance in synthetic and medicinal chemistry has attracted considerable attention in the development of new synthetic strategies for these compounds. In 2013, Sheng et al.18 reported an elegant transformation from internal alkynes into diarylketones using TPPMnCl and oxygen. Despite reports about the cleavage of C–C triple bonds,19 the direct cleavage of C–C triple bonds to form ketones is still unknown and remains both challenging and of great value. As part of our ongoing work to develop organic reactions catalyzed by transition metals,20 we report here a novel copper-catalyzed aerobic synthesis of ketones from internal alkynes.21,22 This reaction constitutes a new transformation from internal alkynes into bisaryl ketones. This protocol also provides a practical, neutral and mild approach for the synthesis of bisaryl ketones (Scheme 1).
In the initial phase of this study, we investigated the reaction of 1,2-diphenylethyne 1a with aniline in the presence K2CO3 (1 equiv.) and CuCl2 (15 mol%) at 120 °C in dimethylsulfoxide (DMSO) under an O2 atmosphere. We were pleased to find that the desired product 2a was isolated at 37% yield after reacting for 16 h (Table 1, entry 1). Various solvents, such as DCE, PhCF3 and DMF, were then screened to determine whether they improved the efficiency of the reaction (Table 1, entries 1–4). DMSO was found to be the most effective solvent for the transformation. DMSO is likely to stabilize the copper catalyst and to assist in the aerobic oxidation process.23 It was found that Cu(OAc)2 was superior to other copper sources (Table 1, entries 5–8). Both Cu(OAc)2 and O2 are essential for the reaction (Table 1, entries 9 and 10). Reactions catalyzed by other transition metals, such as Pd(OAc)2 and NiCl2, did not proceed or gave poorer yields (Table 1, entries 11 and 12). The replacement of aniline with p-toluidine or p-chloroaniline did not affect the reaction efficiency (Table 1, entries 13 and 14). However, the use of n-butylamine did not give the desired product (Table 1, entry 15). Notably, the absence of aniline resulted in no detectable amounts of benzophenone 2a and benzil 3a was formed instead at 81% yield (Table 1, entry 16).
| Entry | Catalyst | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), catalyst (15 mol%), K2CO3 (1 equiv.), aniline (0.6 mmol), solvent (3 mL), 120 °C in O2 atmosphere for 16 h.b Isolated yield.c In Ar atmosphere.d p-Toluidine (0.6 mmol) was used instead of aniline.e p-Chloroaniline (0.6 mmol) was used instead of aniline.f n-Butylamine (0.6 mmol) was used instead of aniline.g Without aniline. | |||
| 1 | CuCl2 | DMSO | 37 |
| 2 | CuCl2 | DCE | Trace |
| 3 | CuCl2 | PhCF3 | Trace |
| 4 | CuCl2 | DMF | 16 |
| 5 | Cu(OAc)2 | DMSO | 72 |
| 6 | CuCl | DMSO | 0 |
| 7 | Cu(OTf)2 | DMSO | 41 |
| 8 | CuBr2 | DMSO | 26 |
| 9c | Cu(OAc)2 | DMSO | 0 |
| 10 | None | DMSO | 0 |
| 11 | Pd(OAc)2 | DMSO | 22 |
| 12 | NiCl2 | DMSO | 0 |
| 13d | Cu(OAc)2 | DMSO | 70 |
| 14e | Cu(OAc)2 | DMSO | 71 |
| 15f | Cu(OAc)2 | DMSO | 0 |
| 16g | Cu(OAc)2 | DMSO | 0 |
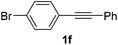
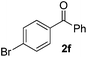
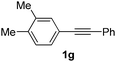
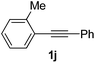
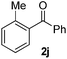
Using this set of optimized conditions, we then investigated a series of internal alkynes to extend the scope of the substrate. As summarized in Table 2, the standard reaction conditions were found to be compatible with a wide range of internal alkynes 1. Different para-substituted 1,2-diarylalkynes could be converted into the corresponding bisaryl ketones in moderate to good yields; electron-donating and electron-withdrawing groups did not have a positive effect on our reaction (Table 2, entries 1–5). It is noteworthy that the polysubstituted 1,2-diarylalkyne gave the desired product 2g with a good yield (Table 2, entry 6). When the meta position was substituted with a methyl- or chloro-group, the product was obtained at 61% and 55% yield, respectively (Table 2, entries 7 and 8). When using the ortho-substituted 1,2-diarylalkyne as the substrate, the corresponding product was formed at 69% yield (Table 2, entry 9). Interestingly, the introduction of heterocyclic molecules into this system made this methodology more useful for the preparation of pharmaceutical products and functional materials (Table 2, entries 10 and 11). The use of substrate 1m with a naphthyl group delivers the desired product at 67% yield (Table 2, entry 12). Apart from the 1,2-diarylalkynes, alkynes bearing one or two aliphatic substituents were unreactive for the copper-catalyzed oxidation reaction (Table 2, entries 13 and 14).
Some control experiments were carried out to probe the mechanism of this transformation. It has been reported that 1,2-diarylalkynes may be oxidized to benzil derivatives.24 Without aniline, benzil 3a was formed at 81% yield (Scheme 2, Reaction (1)). It was found that benzil 3a could give the desired product benzophenone 2a at 87% yield (Scheme 2, Reaction (2)). These results indicate that 1,2-diketones are most likely to be involved in the initial steps of this transformation.
On the basis of these preliminary results and previous studies,17,24–27 the catalytic cycle of this transformation was hypothesized as shown in Scheme 3. 1,2-Diphenylethyne 1a is initially oxidized to produce benzil 3a. The reaction of benzil 3a with aniline then gives the intermediate α-imine ketone A, which quickly converts into the hydrated species B by trapping one molecule of water. Following a benzylic acid rearrangement, B generates intermediate C, which decomposes to the desired product benzophenone 2a.
In summary, the chemoselective oxidative cleavage of the C–C triple bonds of 1,2-diarylalkynes to yield ketones has been described. The application of selective C–C bond cleavage in organic synthesis is an attractive and challenging project. A wide range of 1,2-diarylalkynes can be subjected to this copper-catalyzed reaction under an oxygen atmosphere; the oxidation terminates at the ketone stage. Preliminary mechanistic studies have shown an interesting reaction sequence involving a Wacker-type oxidation/hydration/1,2-aryl migration/C–C bond cleavage. Studies of the mechanism, scope and limitations of this reaction are in progress in our laboratory.
Acknowledgements
We are grateful for financial support from The Educational Bureau of Yunnan Province (2010Y431) and the State Ethnic Affairs Commission (12YNZ05).Notes and references
- Z. Shao and F. Peng, Angew. Chem., Int. Ed., 2010, 49, 9566 CrossRef CAS PubMed.
- M. Chen, X. Zheng, W. Li, J. He and A. Lei, J. Am. Chem. Soc., 2010, 132, 4101 CrossRef CAS PubMed.
- Z. Liu, J. Liu, L. Zhang, P. Liao, J. Song and X. Bi, Angew. Chem., Int. Ed., 2014, 53, 5305 CrossRef CAS PubMed.
- J. Liu, Z. Fang, Q. Zhang, Q. Liu and X. Bi, Angew. Chem., Int. Ed., 2013, 52, 6953 CrossRef CAS PubMed.
- M. Gao, C. He, H. Chen, R. Bai, B. Cheng and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 6958 CrossRef CAS PubMed.
- Q. Lu, J. Zhang, G. Zhao, Y. Qi, H. Wang and A. Lei, J. Am. Chem. Soc., 2013, 135, 11481 CrossRef CAS PubMed.
- M. Oestreich, Sci. Synth., 2005, 25, 199 Search PubMed.
- S. Nishimura, Handbook of heterogeneous catalytic hydrogenation for organic synthesis, Wiley-Interscience, New York, 2001 Search PubMed.
- R. Chinchilla and C. Nájera, Chem. Rev., 2014, 114, 1783 CrossRef CAS PubMed.
- R. He, Z. Huang, Q. Zeng and C. Wang, Angew. Chem., Int. Ed., 2014, 53, 4950 CrossRef CAS PubMed.
- C. He, S. Guo, J. Ke, J. Hao, H. Xu, H. Chen and A. Lei, J. Am. Chem. Soc., 2012, 134, 5766 CrossRef CAS PubMed.
- (a) M. Chen, X. Zheng, W. Li, J. He and A. Lei, J. Am. Chem. Soc., 2010, 132, 4101 CrossRef CAS PubMed; (b) M. Gao, C. He, H. Chen, R. Bai, B. Cheng and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 6958 CrossRef CAS PubMed.
- (a) A. Wang and H. Jiang, J. Am. Chem. Soc., 2008, 130, 5030 CrossRef CAS PubMed; (b) A. S. Dudnik and V. Gevorgyan, Angew. Chem., Int. Ed., 2010, 49, 2096 CrossRef CAS PubMed.
- T. Shen, T. Wang, C. Qin and N. Jiao, Angew. Chem., Int. Ed., 2013, 52, 6677 CrossRef CAS PubMed.
- C. Qin, P. Feng, Y. Ou, T. Shen, T. Wang and N. Jiao, Angew. Chem., Int. Ed., 2013, 52, 7850 CrossRef CAS PubMed.
- M. Li, C. Wang and H. Ge, Org. Lett., 2011, 13, 2062 CrossRef CAS PubMed.
- A. Maji, S. Rana, Akanksha and D. Maiti, Angew. Chem., Int. Ed., 2014, 53, 2428 CrossRef CAS PubMed.
- W. Sheng, Q. Jiang, W. Luo and C. Guo, J. Org. Chem., 2013, 78, 5691 CrossRef CAS PubMed.
- For selected examples of cleavage of C–C triple bonds, see: (a) L. Wang, H. Zhu, L. Lu, F. Yang, X. Liu and Y. Liang, Org. Lett., 2012, 14, 1990 CrossRef CAS PubMed; (b) T. Shimada and Y. Yamamoto, J. Am. Chem. Soc., 2003, 125, 6646 CrossRef CAS PubMed; (c) Y. Liu, F. Song and S. Guo, J. Am. Chem. Soc., 2006, 128, 11332 CrossRef CAS PubMed; (d) C.-H. Jun, H. Lee, C. W. Moon and H.-S. Hong, J. Am. Chem. Soc., 2001, 123, 8600 CrossRef CAS.
- (a) L. Gu and C. Jin, Org. Biomol. Chem., 2012, 10, 7098 RSC; (b) L. Gu, C. Jin, J. Guo, L. Zhang and W. Wang, Chem. Commun., 2013, 49, 10968 RSC; (c) L. Gu, C. Jin, J. Liu, H. Ding and B. Fan, Chem. Commun., 2014, 50, 4643 RSC; (d) L. Gu, J. Liu, L. Zhang, Y. Xiong and R. Wang, Chin. Chem. Lett., 2014, 25, 90 CrossRef CAS PubMed; (e) L. Gu, C. Jin, R. Wang and H. Ding, ChemCatChem, 2014, 6, 1225 CrossRef CAS.
- For selected examples of Cu-catalyzed reactions, see: (a) C. Zhang and N. Jiao, Org. Chem. Front., 2014, 1, 109 RSC; (b) S. Ding, Y. Yan and N. Jiao, Chem. Commun., 2013, 49, 4250 RSC; (c) X. Tang, L. Huang, Y. Xu, J. Yang, W. Wu and H. Jiang, Angew. Chem., Int. Ed., 2014, 53, 4205 CrossRef CAS PubMed; (d) K. Chen, H. J. Chen, J. Wong, J. Yang and S. A. Pullarkat, ChemCatChem, 2013, 5, 3882 CrossRef CAS; (e) M. Wang, Z. Zhang, F. Xie and W. Zhang, Chem. Commun., 2014, 50, 3163 RSC; (f) Q. Sha and Y. Wei, ChemCatChem, 2013, 6, 131 CrossRef; (g) F. Ye, X. Ma, H. Li, Y. Zhang and J. Wang, J. Am. Chem. Soc., 2012, 134, 5742 CrossRef CAS PubMed; (h) J. Gallardo-Donaire and R. Martin, J. Am. Chem. Soc., 2013, 135, 9350 CrossRef CAS PubMed; (i) J. Liu, W. Chen and L. Wang, RSC Adv., 2013, 3, 4723 RSC; (j) Y. Yang, H. Ren, D. Wang, F. Shi and C. Wu, RSC Adv., 2013, 3, 10434 RSC.
- For selected examples of aerobic oxidation reactions, see: (a) H. Tian, H. Qiao, C. Zhu and H. Fu, RSC Adv., 2014, 4, 2694 RSC; (b) Q. Wang and S. L. Schreiber, Org. Lett., 2009, 11, 5178 CrossRef CAS PubMed; (c) Q. Cao, L. M. Dornan, L. Rogan, N. L. Hughes and M. J. Muldoon, Chem. Commun., 2014, 50, 4524 RSC; (d) S. Wertz and A. Studer, Green Chem., 2013, 15, 3116 RSC; (e) Y. Liang and N. Jiao, Angew. Chem., Int. Ed., 2014, 53, 548 CrossRef CAS PubMed; (f) Z. Xu, C. Zhang and N. Jiao, Angew. Chem., Int. Ed., 2012, 51, 11367 CrossRef CAS PubMed; (g) Y. Su, X. Sun, G. Wu and N. Jiao, Angew. Chem., Int. Ed., 2013, 52, 9808 CrossRef CAS PubMed; (h) J. Liu, Q. Liu, H. Yi, C. Qin, R. Bai, X. Qi, Y. Lan and A. Lei, Angew. Chem., Int. Ed., 2014, 53, 502 CrossRef CAS PubMed; (i) X. Tang, L. Huang, C. Qi, W. Wu and H. Jiang, Chem. Commun., 2013, 49, 9597 RSC.
- L. Zhang, X. Bi, X. Guan, X. Li, Q. Liu, B. Barry and P. Liao, Angew. Chem., Int. Ed., 2013, 52, 11303 CrossRef CAS PubMed.
- S. Enthaler, ChemCatChem, 2011, 3, 1929 CrossRef CAS.
- M. V. Bhatt, Tetrahedron, 1964, 20, 803 CrossRef CAS.
- J. Hine and H. W. Haworth, J. Am. Chem. Soc., 1958, 80, 2274 CrossRef CAS.
- C. M. Comisar and P. E. Savage, Green Chem., 2005, 7, 800 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c4ra06460g |
| This journal is © The Royal Society of Chemistry 2015 |