Continuous l-lactic acid production from defatted rice bran hydrolysate using corn stover bagasse immobilized carrier
Abstract
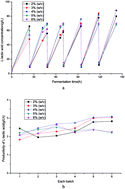
In this paper, L-lactic acid (LLA) was produced using defatted rice bran hydrolysate. Agricultural waste corn stover bagasse (CSB), under the conditions of good mechanical and chemical stabilities, was used as the immobilized carrier. As a result, a maximal concentration of 88 g L−1 of LLA with an average yield of 0.95 g g−1 and productivity of 5.20 g L−1 h−1 were achieved in a single-stage immobilized repeated-batch fermentation. In addition, when single-stage immobilized continuous fermentation was carried out, a maximal LLA concentration of 84 g L−1 was obtained with an average yield and productivity of 0.97 g g−1 and 5.73 g L−1 h−1, respectively. To balance the increases in the productivity, concentration and yield of LLA, two-stage immobilized continuous fermentation was performed. LLA productivities of 6.20 g L−1 h−1 in the first stage and 2.18 g L−1 h−1 in the second stage were achieved. The whole immobilized fermentation process has potential in L-lactic acid biorefineries and industrialization.

 Please wait while we load your content...
Please wait while we load your content...