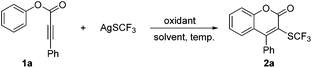
Direct radical trifluoromethylthiolation and thiocyanation of aryl alkynoate esters: mild and facile synthesis of 3-trifluoromethylthiolated and 3-thiocyanated coumarins†
Yao-Fu
Zeng
,
Dong-Hang
Tan
,
Yunyun
Chen
,
Wen-Xin
Lv
,
Xu-Ge
Liu
,
Qingjiang
Li
and
Honggen
Wang
*
School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510006, China. E-mail: wanghg3@mail.sysu.edu.cn
First published on 16th September 2015
Abstract
A mild and convenient oxidative radical cyclization of aryl alkynoate esters for the synthesis of 3-trifluoromethylthiolated and 3-thiocyanated coumarins has been developed using AgSCF3 and AgSCN as the corresponding radical sources, respectively. This protocol is characterized by readily available starting materials, excellent functional group tolerance and good yields.
Introduction
Coumarins represent a privileged class of structural scaffolds in medicinal chemistry due to their wide spectrum of biological activities, such as anti-HIV,1 antibacterial,2 anti-inflammatory,3 antitumor,4 and antimalarial activities.5 As such, the development of efficient methods for the construction of structurally diverse coumarins bearing valuable functional groups from easily accessible starting materials is in high demand. Recently, the tandem functionalization/cyclization of aryl alkynoate esters has emerged as a powerful strategy to synthesize 3-functionalized coumarins.6 For instance, it was reported that 3-trifluoromethylated coumarins could be prepared by the reaction of aryl alkynoate esters with Togni's reagent following a radical addition/cyclization mechanism.6a Similarly, various 3-substituted coumarins have been accessed via radical alkyne sulfonation,6b phosphorylation,6c acylation,6d–f and difluoroacetylation6f/cyclization pathways under oxidative or redox-neutral reaction conditions. Herein, we report our realization of a mild and facile synthesis of 3-trifluoromethylthiolated and thiocyanated coumarins by using AgSCF3 and AgSCN as the radical sources, respectively. A broad substrate scope and good functional group tolerance were observed.Due to the typically desirable strong electron-withdrawing effect and high lipophilicity properties, the trifluoromethylthio group is frequently incorporated into bioactive compounds.7 Therefore, numerous trifluoromethylthiolation approaches have been developed by using either nucleophilic8 or electrophilic SCF3 reagents.9 Nevertheless, the introduction of the SCF3 group through a radical pathway has been relatively less explored.10–15 Recently, Wang disclosed the synthesis of SCF3-containing oxindoles through a ˙SCF3 addition/cyclization pathway.11 Wang and Xu reported that an intramolecular oxytrifluoromethylthiolation of alkenes is feasible under the catalysis of copper.12 Silver-meditated direct trifluoromethylthiolation of C(sp3)–H bonds have been developed by Tang13 and Chen,14 respectively. Very recently, Liang disclosed an AgSCF3-mediated radical cascade cyclization/trifluoromethylthiolation of 1,6-enynes.15
On the other hand, the thiocyano group is a versatile synthon which could be readily converted into other functional groups, such as sulfide,16 thiocarbamate,17 trifluoromethylthio18 and thiotetrazole.19 A variety of thiocyanation methods with the involvement of SCN are known.20 However, the tandem thiocyanation/cyclization reactions of unsaturated C–C bonds remain less explored.21 In particular, no precedent exists for thiocyanation/cyclization reactions of alkynes.
Results and discussion
Initially, we investigated the reaction of phenyl 3-phenylpropiolate (1a) with AgSCF3 using K2S2O8 as the oxidant in the presence of HMPA at 75 °C under argon (Table 1, entry 1).11 To our delight, the desired cyclized 3-trifluoromethylthiolated coumarin 2a was obtained in 25% yield. Further screening of solvents showed that DMSO was superior to others, increasing the yield to 42% (entries 1–5). When HMPA was removed from the reaction mixture, the yield was improved to 57% (entry 6). The increasing of reaction temperature to 100 °C gave a similar yield (entry 7). However, a lower temperature of 30 °C gave an increased yield of 71% (entry 8). Next, the effects of various oxidants such as oxone, PhI(OAc)2, and Cu(OAc)2·H2O were investigated (entries 9–11). Disappointedly, none of them were effective for the reaction. By increasing the loading of K2S2O8 (4.0 equiv.) and AgSCF3 (2.0 equiv.), the yield was further improved to 78% (entry 12). A slightly lower yield of 74% was observed when the reaction was performed under air (entry 13). Additional control experiments demonstrated that K2S2O8 was essential for the reaction as its omission gave no desired product (entry 14).| Entry | Oxidant (equiv.) | Solvent | Additive (equiv.) | Temp (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Unless otherwise noted, all reactions were conducted with 1a (0.1 mmol), AgSCF3 (1.5 equiv.), and oxidant (3.0 equiv.) in solvent (1.0 mL) at 30 °C for 15 h under Ar. b 1H NMR yield based on 1a using methyl 4-bromobenzoate as the internal standard. c Isolated yield. d Oxidant (4.0 equiv.), AgSCF3 (2.0 equiv.). e The reaction was performed under air. | |||||
| 1 | K2S2O8 (3.0) | CH3CN | HMPA (0.5) | 75 | 25c |
| 2 | K2S2O8 (3.0) | DMSO | HMPA (0.5) | 75 | 42 |
| 3 | K2S2O8 (3.0) | Toluene | HMPA (0.5) | 75 | 0 |
| 4 | K2S2O8 (3.0) | DCE | HMPA (0.5) | 75 | Trace |
| 5 | K2S2O8 (3.0) | DMF | HMPA (0.5) | 75 | 20 |
| 6 | K2S2O8 (3.0) | DMSO | — | 75 | 57 |
| 7 | K2S2O8 (3.0) | DMSO | — | 100 | 58 |
| 8 | K2S2O8 (3.0) | DMSO | — | 30 | 71 |
| 9 | Oxone (3.0) | DMSO | — | 30 | Trace |
| 10 | PhI(OAc) (3.0) | DMSO | — | 30 | Trace |
| 11 | Cu(OAc)2·H2O (3.0) | DMSO | — | 30 | 0 |
| 12 | K 2 S 2 O 8 (4.0) | DMSO | — | 30 | 78 |
| 13d,e | K2S2O8 (4.0) | DMSO | — | 30 | 74 |
| 14 | — | DMSO | — | 30 | 0 |
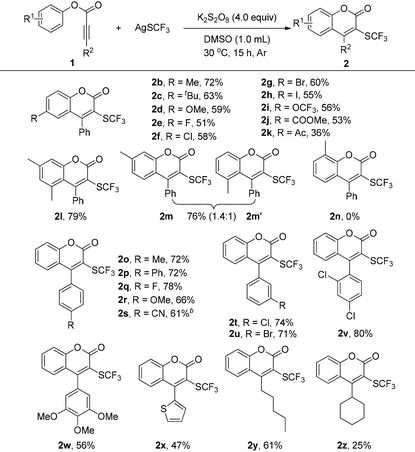
With the optimized conditions in hand, various substituted aryl alkynoates 1a–z were synthesized and subjected to evaluate the scope and limitation of this transformation. As depicted in Table 2, alkynoates with different substituents on the phenoxy ring, regardless of the electron-withdrawing or -donating properties, could be converted to the corresponding products in moderate to good yields (2a–m). A variety of functional groups, such as methoxyl (2d), halogen (2e–h), trifluoromethoxyl (2i), ester (2j), and acetyl (2k) were well tolerated under the reaction conditions. Interestingly, the di-meta-substituted substrate did not hamper the reactivity, giving a good yield of 79% (2l). Two regioisomers 2m and 2m‘ were obtained in a ratio of 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 when a meta-methyl substituted phenoxy ring was employed. No desired product was detected with a methyl group substituted at the ortho-position of the phenoxy (2n). Next, the compatibility of the substituents on the alkynyl moiety was also investigated (2o–w). Arylpropilates with substituents at the ortho-, meta-, or para-position of the phenyl ring were all well tolerated in the reaction, affording the products in moderate to good yields. The heterocyclic 2-thienyl group was also compatible (2x). Delightedly, phenyl 2-octynoate (2y) and phenyl 3-cyclohexylpropiolate (2z) bearing an alkyl substituent were also suitable substrates, thus greatly extending the scope of this transformation.
1 when a meta-methyl substituted phenoxy ring was employed. No desired product was detected with a methyl group substituted at the ortho-position of the phenoxy (2n). Next, the compatibility of the substituents on the alkynyl moiety was also investigated (2o–w). Arylpropilates with substituents at the ortho-, meta-, or para-position of the phenyl ring were all well tolerated in the reaction, affording the products in moderate to good yields. The heterocyclic 2-thienyl group was also compatible (2x). Delightedly, phenyl 2-octynoate (2y) and phenyl 3-cyclohexylpropiolate (2z) bearing an alkyl substituent were also suitable substrates, thus greatly extending the scope of this transformation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
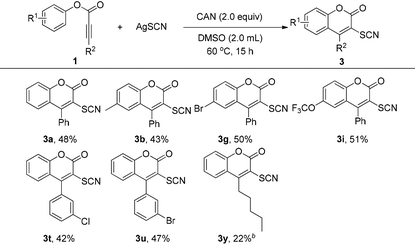
Encouraged by the above results, we then turned our attention to investigate the feasibility of thiocyanation of alkynoates using a similar protocol (Table 3). To our disappointment, the desired 3-thiocyanated coumarin products were not produced under the above standard conditions using AgSCN as the thiocyano source. It was reported that the thiocyanate radical could be formed by the oxidation of a thiocyanate anion with various oxidants, such as CAN (Cerium (IV) ammonium nitrate), hypervalent iodine reagents, oxone, and so on.20 Therefore, screening of different oxidants and thiocyanate salts proved that the combination of CAN (Ce(NH4)2(NO3)6, 2.0 equiv.) and silver thiocyanate (2.0 equiv.) in DMSO (2.0 mL) at 60 °C was effective for the reaction, affording the product in 48% yield (3a). Similar to the trifluoromethylthiolation reaction, aryl alkynoates with various substituents were also compatible with the reaction conditions to give the corresponding products in moderate yields. Phenyl 2-octynoate could also be converted to the cyclization product with CH3CN as the solvent, albeit in a low yield of 22% (3y).
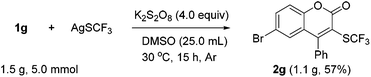
To demonstrate the scalability of our protocol, the reaction of 1g on the 5 mmol scale was conducted under the standard conditions, giving 1.1 gram of the product in 57% yield (Scheme 1).
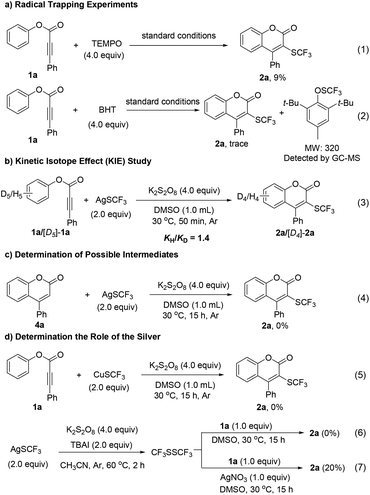
To probe the possible reaction mechanism, a series of control experiments were carried out (Scheme 2). Firstly, radical trapping experiments were conducted. When 4.0 equiv. of TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) was added, only 9% yield was detected (eqn (1)). Additionally, BHT (butylhydroxytoluene) also inhibited the transformation significantly and the BHT–SCF3 (MS = 320) adduct was detected by GC-MS analysis (eqn (2)), indicating a radical pathway involved and that a ˙SCF3 radical might be generated in the reaction. An intermolecular kinetic isotope effect (KIE) value of 1.4 suggested the C–H bond cleavage step was not rate-determining (eqn (3)). To determine the possible intermediate of this transformation, coumarin 4a was subjected to the standard conditions. Trifluoromethylthiolation coumarin 2a could not be obtained (eqn (4)). Finally, the role of silver in the reaction system was studied. No product was formed when AgSCF3 was replaced by CuSCF3, which indicated that silver was essential for the reactivity (eqn (5)). It was reported that F3CSSCF3 might act as the ˙SCF3 source in some reactions.11 Therefore, F3CSSCF3 was prepared according to a known procedure22 and used for our reaction. In the presence of AgNO3, the trifluoromethylthiolation product was obtained in 20% yield (eqn (7)), while no desired product was observed without silver (eqn (6)).
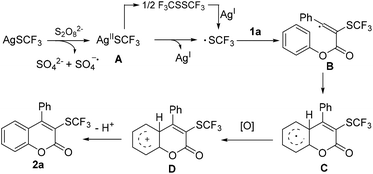
Based on the above observations and previous reports,6,11–15,23 a plausible mechanism was outlined in Scheme 3. Initially, AgSCF3 is oxidized by K2S2O8 to generate [AgIISCF3] species A, which could produce the ˙SCF3 radical through single electron transfer. Alternatively, F3CSSCF3, generated from [AgIISCF3], might be a possible source of ˙SCF3. Regio-selective addition of the ˙SCF3 to 1a affords vinyl radical B. Thereafter, an intramolecular cyclization gives radical intermediate C, which is further oxidized by AgII to deliver F–C reaction Wheland intermediate D. The deprotonation/rearomatization yields final product 2a.
Conclusions
In conclusion, we have developed a novel oxidative radical cyclization of aryl alkynoate esters. This method provides a simple and efficient synthesis of various 3-trifluoromethylthiolated or 3-thiocyanated coumarins from readily accessible starting materials. Mild reaction conditions, excellent functional group tolerance, and generally good yields were observed. Preliminary mechanistic studies suggested that a radical reaction pathway is involved.Acknowledgements
We are grateful for the support of this work by the “1000-Youth Talents Plan”, a Start-up Grant from Sun Yat-sen University and the National Natural Science Foundation of China (81402794 and 21472250).Notes and references
- (a) T. Pengsuparp, M. Serit, S. H. Hughes, D. D. Soejarto and J. M. Pezzuto, J. Nat. Prod., 1996, 59, 839 CrossRef CAS PubMed; (b) E. B. B. Ong, N. Watanabe, A. Saito, Y. Futamura, K. H. Abd EI Galil, A. Koito, N. Najimudin and H. Osada, J. Biol. Chem., 2011, 286, 14049 CrossRef CAS PubMed.
- T. Taechowisan, C. Lu, Y. Shen and S. Lumyong, Microbiology, 2005, 151, 1691 CrossRef CAS PubMed.
- T. Taechowisan, C. Lu, Y. Shen and S. Lumyong, Food Agric. Immunol., 2007, 18, 203 CrossRef CAS PubMed.
- (a) M. Itoigawa, C. Ito, H. T.-W. Tan, M. Kuchide, H. Tokuda, H. Nishino and H. Furukawa, Cancer Lett., 2001, 169, 15 CrossRef CAS; (b) C. Rappl, P. Barbier, V. Bourgarel-Rey, C. Gregoire, R. Gilli, M. Carre, S. Combes, J. Finet and V. Peyrot, Biochemistry, 2006, 45, 9210 CrossRef CAS PubMed; (c) C. Bailly, C. Bal, P. Barbier, S. Combes, J. Finet, M. Hildebrand, V. Peyrot and N. Wattez, J. Med. Chem., 2003, 46, 5437 CrossRef CAS PubMed.
- R. Argotte-Ramos, G. Ramírez-Avila, M. C. Rodríguez-Gutiérrez, M. Ovilla-Muñoz, H. Lanz-Mendoza, M. H. Rodríguez, M. González-Cortazar and L. Alvarez, J. Nat. Prod., 2006, 69, 1442 CrossRef CAS PubMed.
- (a) Y. Li, Y. Lu, G. Qiu and Q. Ding, Org. Lett., 2014, 16, 4240 CrossRef CAS PubMed; (b) W. Wei, J. Wen, D. Yang, M. Guo, Y. Wang, J. You and H. Wang, Chem. Commun., 2015, 51, 768 RSC; (c) X. Mi, C. Wang, M. Huang, J. Zhang, Y. Wu and Y. Wu, Org. Lett., 2014, 16, 3356 CrossRef CAS PubMed; (d) X. Mi, C. Wang, M. Huang, Y. Wu and Y. Wu, J. Org. Chem., 2015, 80, 148 CrossRef CAS PubMed; (e) K. Yan, D. Yang, W. Wei, F. Wang, Y. Shuai, Q. Li and H. Wang, J. Org. Chem., 2015, 80, 1550 CrossRef CAS PubMed; (f) T. Liu, Q. Ding, Q. Zong and G. Qiu, Org. Chem. Front., 2015, 2, 670 RSC; (g) W. Fu, M. Zhu, G. Zou, C. Xu, Z. Wang and B. Ji, J. Org. Chem., 2015, 80, 4766 CrossRef CAS PubMed.
- (a) B. Manteau, S. Pazenok, J.-P. Vors and F. R. Leroux, J. Fluorine Chem., 2010, 131, 140 CrossRef CAS PubMed; (b) F. Leroux, P. Jeschke and M. Schlosser, Chem. Rev., 2005, 105, 827 CrossRef CAS PubMed; (c) A. Leo, C. Hansch and D. Elkins, Chem. Rev., 1971, 71, 525 CrossRef CAS; (d) X.-H. Xu, K. Matsuzaki and N. Shibata, Chem. Rev., 2015, 115, 731 CrossRef CAS PubMed.
- (a) C. Chen, Y. Xie, L. Chu, R.-W. Wang, X. Zhang and F.-L. Qing, Angew. Chem., Int. Ed., 2012, 51, 2492 CrossRef CAS PubMed; (b) Q. Xiao, J. Sheng, Q. Ding and J. Wu, Eur. J. Org. Chem., 2014, 217 CrossRef CAS PubMed; (c) M. Jiang, F. Zhu, H. Xiang, X. Xu, L. Deng and C. Yang, Org. Biomol. Chem., 2015, 13, 6935 RSC; (d) C. Chen, X.-H. Xu, B. Yang and F.-L. Qing, Org. Lett., 2014, 16, 3327 Search PubMed; (e) M. Hu, J. Rong, W. Miao, Y. Ni, Y. Han and J. Hu, Org. Lett., 2014, 16, 2030 CrossRef CAS PubMed; (f) D. Kong, Z. Jiang, S. Xin, Z. Bai, Y. Yuan and Z. Weng, Tetrahedron, 2013, 69, 6046 CrossRef CAS PubMed; (g) C. Chen, L. Chu and F.-L. Qing, J. Am. Chem. Soc., 2012, 134, 12454 CrossRef CAS PubMed; (h) L. D. Tran, I. Popov and O. Daugulis, J. Am. Chem. Soc., 2012, 134, 18237 CrossRef CAS PubMed; (i) G. Danoun, B. Bayarmagnai, M. F. Gruenberg and L. J. Goossen, Chem. Sci., 2014, 5, 1312 RSC; (j) Z. Weng, W. He, C. Chen, R. Lee, D. Tan, Z. Lai, D. Kong, Y. Yuan and K.-W. Huang, Angew. Chem., Int. Ed., 2013, 52, 1548 CrossRef CAS PubMed; (k) C.-P. Zhang and D. A. Vicic, J. Am. Chem. Soc., 2012, 134, 183 CrossRef CAS PubMed; (l) J. Xu, X. Mu, P. Chen, J. Ye and G. Liu, Org. Lett., 2014, 16, 3942 CrossRef CAS PubMed; (m) M. Rueping, N. Tolstoluzhsky and P. Nikolaienko, Chem. – Eur. J., 2013, 19, 14043 CrossRef CAS PubMed; (n) K.-P. Wang, S. Y. Yun, P. Mamidipalli and D. Lee, Chem. Sci., 2013, 4, 3205 RSC; (o) G. Teverovskiy, D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2011, 50, 7312 CrossRef CAS PubMed; (p) K. Zhang, J.-B. Liu and F.-L. Qing, Chem. Commun., 2014, 50, 15999 RSC.
- For review, see: (a) X. Shao, C. Xu, L. Lu and Q. Shen, Acc. Chem. Res., 2015, 48, 1227 CrossRef CAS PubMed. For selected examples, see: (b) X. Wang, T. Yang, X. Cheng and Q. Shen, Angew. Chem., Int. Ed., 2013, 52, 12860 CrossRef CAS PubMed; (c) K. Kang, C. Xu and Q. Shen, Org. Chem. Front., 2014, 1, 294 RSC; (d) S. Alazet, L. Zimmer and T. Billard, Chem. – Eur. J., 2014, 20, 8589 CrossRef CAS PubMed; (e) X. Shao, C. Xu, L. Lu and Q. Shen, J. Org. Chem., 2015, 80, 3021 Search PubMed; (f) R. Pluta, P. Nikolaienko and M. Rueping, Angew. Chem., Int. Ed., 2014, 53, 1650 CrossRef CAS PubMed; (g) B. Ma, X. Shao and Q. Shen, J. Fluorine Chem., 2015, 171, 73 CrossRef CAS PubMed; (h) J. Sheng, C. Fan and J. Wu, Chem. Commun., 2014, 50, 5494 RSC; (i) M. Rueping, X. Liu, T. Bootwicha, R. Pluta and C. Merkens, Chem. Commun., 2014, 50, 2508 RSC; (j) Y. Li, G. Li and Q. Ding, Eur. J. Org. Chem., 2014, 5017 CrossRef CAS PubMed; (k) X. Shao, X. Wang, T. Yang, L. Lu and Q. Shen, Angew. Chem., Int. Ed., 2013, 52, 3457 CrossRef CAS PubMed; (l) E. V. Vinogradova, P. Müller and S. L. Buchwald, Angew. Chem., Int. Ed., 2014, 53, 3125 CrossRef CAS PubMed; (m) C. Xu and Q. Shen, Org. Lett., 2014, 16, 2046 CrossRef CAS PubMed; (n) C. Xu, B. Ma and Q. Shen, Angew. Chem., Int. Ed., 2014, 53, 9316 CrossRef CAS PubMed; (o) Y.-D. Yang, A. Azuma, E. Tokunaga, M. Yamasaki, M. Shiro and N. Shibata, J. Am. Chem. Soc., 2013, 135, 8782 CrossRef CAS PubMed; (p) F. Baert, J. Colomb and T. Billard, Angew. Chem., Int. Ed., 2012, 51, 10382 CrossRef CAS PubMed; (q) S. Alazet, L. Zimmer and T. Billard, Angew. Chem., Int. Ed., 2013, 52, 10814 CrossRef CAS PubMed; (r) Y. Yang, X. Jiang and F.-L. Qing, J. Org. Chem., 2012, 77, 7538 CrossRef CAS PubMed; (s) J. Sheng, S. Li and J. Wu, Chem. Commun., 2014, 50, 578 RSC; (t) J. Liu, L. Chu and F.-L. Qing, Org. Lett., 2013, 15, 894 CrossRef CAS PubMed; (u) Q. Xiao, J. Sheng, Z. Chen and J. Wu, Chem. Commun., 2013, 49, 8647 RSC.
- (a) J. F. Harris, J. Am. Chem. Soc., 1962, 84, 3148 CrossRef CAS; (b) J. F. Harris and F. W. Stacey, J. Am. Chem. Soc., 1961, 83, 840 CrossRef CAS; (c) S. Andreades, J. F. Harris and W. A. Sheppard, J. Org. Chem., 1964, 29, 898 CrossRef CAS; (d) N. Fuentes, W. Kong, L. Fernández-Sánchez, E. Merino and C. Nevado, J. Am. Chem. Soc., 2015, 137, 964 CrossRef CAS PubMed.
- F. Yin and X. Wang, Org. Lett., 2014, 16, 1128 CrossRef CAS PubMed.
- L. Zhu, G. Wang, Q. Guo, Z. Xu, D. Zhang and R. Wang, Org. Lett., 2014, 16, 5390 CrossRef CAS PubMed.
- S. Guo, X. Zhang and P. Tang, Angew. Chem., Int. Ed., 2015, 54, 4065 CrossRef CAS PubMed.
- H. Wu, Z. Xiao, J. Wu, Y. Guo, J.-C. Xiao, C. Liu and Q.-Y. Chen, Angew. Chem., Int. Ed., 2015, 54, 4070 CrossRef CAS PubMed.
- Y.-F. Qiu, X.-Y. Zhu, Y.-X. Li, Y.-T. He, F. Yang, J. Wang, H.-L. Hua, L. Zheng, L.-C. Wang, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2015, 17, 3694 CrossRef CAS PubMed.
- (a) T. Billard, B. R. Langlois and M. Médebieele, Tetrahedron Lett., 2001, 42, 3463 CrossRef CAS; (b) P. A. Grieco, Y. Yokoyama and E. Williams, J. Org. Chem., 1978, 43, 1283 CrossRef CAS; (c) B. Das, V. S. Reddy and M. Krishnaiah, Tetrahedron Lett., 2006, 47, 8471 CrossRef CAS PubMed.
- (a) R. Riemschneider, J. Am. Chem. Soc., 1956, 78, 3148 CrossRef; (b) R. Riemschneider, F. Wojahn and G. Orlick, J. Am. Chem. Soc., 1951, 73, 5905 CrossRef.
- T. Billard, S. Large and B. R. Langlois, Tetrahedron Lett., 1997, 38, 65 CrossRef CAS.
- S. Vorona, T. Artamonova, Y. Zevatskii and L. Myznikov, Synthesis, 2014, 781 Search PubMed.
- (a) A. D. Mico, R. Margarita, A. Mariani and G. Piancatelli, Tetrahedron Lett., 1996, 37, 1889 CrossRef; (b) V. Nair and L. G. Nair, Tetrahedron Lett., 1998, 39, 4583 Search PubMed; (c) V. Nari, T. G. George, L. G. Nair and S. B. Panicker, Tetrahedron Lett., 1999, 40, 1195 CrossRef; (d) G. Wu, Q. Liu, Y. Shen, W. Wu and L. Wu, Tetrahedron Lett., 2005, 46, 5831 CrossRef CAS PubMed; (e) X. Pan, M. Lei, J.-P. Zou and W. Zhang, Tetrahedron Lett., 2009, 50, 347 CrossRef CAS PubMed; (f) W. Fan, Q. Yang, F. Xu and P. Li, J. Org. Chem., 2014, 79, 10588 CrossRef CAS PubMed.
- H. Yang, X.-H. Duan, J.-F. Zhao and L.-N. Guo, Org. Lett., 2015, 17, 1998 CrossRef CAS PubMed.
- (a) R. E. A. Dear and E. E. Gilbert, J. Fluorine Chem., 1974, 4, 107 CrossRef CAS; (b) G. Haran and D. W. A. Sharp, Chem. Soc., Perkin Trans. 1, 1972, 34 RSC.
- (a) A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2011, 50, 5018 CrossRef CAS PubMed; (b) Y. Fujiwara, V. Domingo, I. B. Seiple, R. Gianatassio, M. D. Bel and P. S. Bara, J. Am. Chem. Soc., 2011, 133, 3292 CrossRef CAS PubMed; (c) N. R. Patel and R. A. Flowers II, J. Am. Chem. Soc., 2013, 135, 4672 CrossRef CAS PubMed; (d) R. Wang and J. R. Falck, Org. Chem. Front., 2014, 1, 1029 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1409264. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00271k |
| This journal is © the Partner Organisations 2015 |