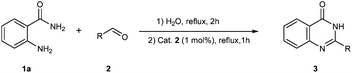
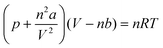Acceptorless dehydrogenative condensation of o-aminobenzamides with aldehydes to quinazolinones in water catalyzed by a water-soluble iridium complex [Cp*Ir(H2O)3][OTf]2†
Feng
Li
*ab,
Lei
Lu
a and
Juan
Ma
a
aSchool of Chemical Engineering, Nanjing University of Science & Technology, Nanjing 210094, P. R. China. E-mail: fengli@njust.edu.cn; Fax: +86-25-84431939; Tel: +86-25-84317316
bState Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, P. R. China
First published on 6th October 2015
Abstract
A general and efficient method for the synthesis of quinazolinones via acceptorless dehydrogenative condensation of o-aminobenzamides with aldehydes in water has been accomplished. In the presence of [Cp*Ir(H2O)3][OTf]2, a variety of desirable products were obtained in high yields with high atom economy under environmentally benign conditions. Notably, this research will facilitate the progress of acceptorless dehydrogenative reactions in water catalyzed by water-soluble organometallic complexes.
Introduction
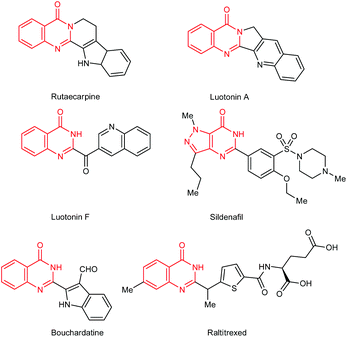
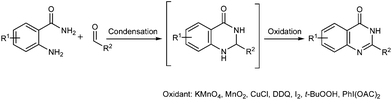
Quinazolinones represent a class of privileged scaffolds that occur in approximately 150 naturally occurring alkaloids, such as rutaecarpine, luotonin A, luotonin F, sildenafil, bouchardatine and raltitrexed (Scheme 1).1 They also exhibit a wide range of biological and pharmacological activities, including antibacterial,2 antifungal,3 antiviral,4 antiinflammatory,5 anticonvulsant,6 antimalarial7 and anticancer properties.8 Although numerous methods have been developed,9 the most classical and general protocols for the synthesis of quinazolinones are still through the condensation between o-aminobenzamides and aldehydes followed by the oxidation of the resulting aminal intermediates (Scheme 2).10 However, these procedures suffer from the use of stoichiometric or excess amounts of toxic and/or hazardous oxidants, such as KMnO4,10a MnO2,10b CuCl,10c DDQ,10d I2,10et-BuOOH10f and PhI(OAc)2,10g and the generation of a large amount of harmful byproducts. In 2012, Mulakayala, Oruganti and co-workers reported the synthesis of quinazolinones from o-aminobenzamides with aldehydes in the presence of InCl3 (10 mol%) without an additional oxidant and oxygen gas may work as the oxidant.11 In addition, the above procedures were generally performed in organic solvents, which might cause environmental pollution.In recent years, transition-metal-catalyzed acceptorless dehydrogenative oxidation with the liberation of hydrogen gas has attracted much attention because it represents a clean and atom economical strategy instead of traditional oxidation reactions.12 The liberated hydrogen gas is also regarded as one of the most promising energies of the future.13 Significant advances include dehydrogenative oxidation of alcohols,14 nitrogen-containing heterocycles,15 amines to nitriles,16 C–C single bonds adjacent to functional groups to form α,β-unsaturated compounds.17 On the other hand, the development of organic synthesis in water has become increasingly important because water is a cheap, safe and environmentally benign solvent compared with organic solvents.18 Despite these advances, acceptorless dehydrogenative oxidation in water catalyzed by water-soluble organometallic complexes remains less explored. Recently, Fujita, Yamaguchi and co-workers demonstrated the dehydrogenative oxidation of alcohols to carbonyl compounds and dehydrogenative lactonization of diols in water catalyzed by a water-soluble bifunctional iridium complex [Cp*Ir(6,6′-(OH)2bpy)(H2O)][OTf]2 (Cp* = η5-pentamethylcyclopentadienyl, bpy = 2,2′-bipyridine).19 From both synthetic and environmental points of view, the development of an efficient homogeneous catalytic system for the acceptorless dehydrogenative oxidation of nitrogen-containing heterocycles in water is apparently highly desirable.
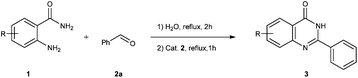
We have reported a series of iridium-catalyzed C–N and C–C bond-forming reactions based on the activation of alcohols as electrophiles.20 In 2014, we demonstrated the rearrangement of aldoximes to amides21a and the N-alkylation of sulfonamides with alcohols to N-alkylated sulfonamides21b in water catalyzed by water-soluble iridium complexes. As part of our continuing interest in the development of iridium-catalyzed reactions in water, we herein wish to report the acceptorless dehydrogenative condensation of o-aminobenzamides with aldehydes to quinazolinones in water catalyzed by a water-soluble iridium complex.
Results and discussion
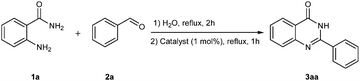
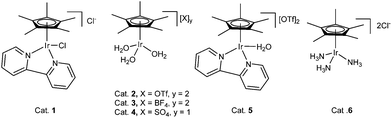
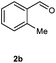
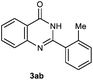
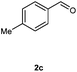
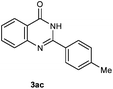
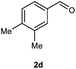
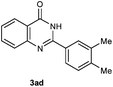
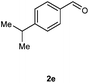
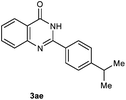
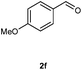
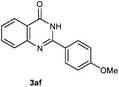
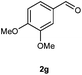
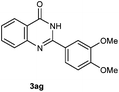
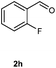
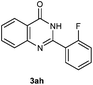
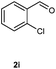
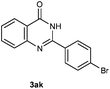
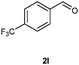
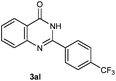
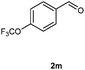
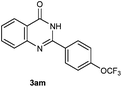
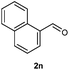
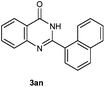
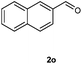
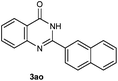
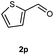
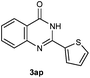
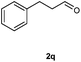
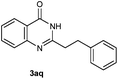
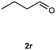
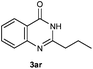
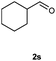
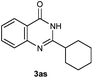
Our initial investigation focused on the reaction of o-aminobenzamide (1a) with benzaldehyde (2a) in water. As shown in Table 1, several different types of water-soluble Cp*Ir complexes were assayed for their catalytic ability towards this model reaction. In a typical procedure, the reaction of 1a and 2a was refluxed in water for 2 h and the refluxing of the reaction mixture was continued for 1 h after a catalyst (1 mol%) was added. In the presence of a cationic iridium complex [Cp*Ir(bpy)Cl]Cl (bpy = 2,2′-bipyridine) (Cat. 1), the reaction afforded the desired product 3aa in 83% yield (Table 1, entry 1). When Cp*Ir complexes bearing more aqua ligands [Cp*Ir(H2O)3][OTf]2 (Cat. 2), [Cp*Ir(H2O)3][BF4]2 (Cat. 3) and [Cp*Ir(H2O)3][SO4] (Cat. 4) were used as the catalyst, the product 3aa was obtained in 80–89% yields (Table 1, entries 2–4). Using [Cp*Ir(bpy)(H2O)][OTf]2 (Cat. 5) as an alternative catalyst, this reaction gave the product 3aa in only 30% yield (Table 1, entry 5). The Cp*Ir complex bearing three ammonia ligands [Cp*Ir(NH3)3][Cl]2 (Cat. 6) was also screened, and the desired product 3aa could be obtained in 85% yield (Table 1, entry 6). Apparently, apart from Cat. 5, other tested catalysts exhibited high catalytic activities. Among them, [Cp*Ir(H2O)3][OTf]2 is the most effective for this transformation.Having established the optimal reaction conditions (Table 1, entry 2), the scope of reaction with respect to aldehydes was investigated and the results are shown in Table 2. Reactions with benzaldehydes bearing one or two electron-donating groups, such as methyl (2b–c), dimethyl (2d), isopropyl (2e), methoxy (2f) and dimethoxy (2g), gave the corresponding products 3ab–3ag in 82–91% yields (Table 2, entries 1–6). Similarly, benzaldehydes bearing a halogen atom, such as fluoro (2h), chloro (2i–j) and bromo (2k), were converted to the desired products 3ah–3ak in 78–87% yield (Table 2, entries 7–10). Benzaldehydes bearing a strong electron-withdrawing group, such as trifluoromethyl (2l) and trifluoromethoxy (2m), were also proven to be suitable substrates and the desired products 3al and 3am could be obtained in 81% and 78% yields, respectively (Table 2, entries 11 and 12). Furthermore, high catalytic activities were found in transformations of 1-naphthaldehyde (2n), 2-naphthaldehyde (2o) and 2-thiophenaldehyde (2p) to the corresponding products 3an–3ap (Table 2, entries 13–15). In the case of aliphatic aldehydes, such as phenylpropyl aldehyde (2q), butyraldehyde (2r) and cyclohexanecarbaldehyde (2s), the desired products 3aq–3as could be obtained in 81–84% yields as well (Table 2, entries 16–18).
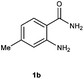
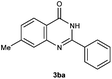
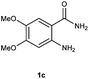
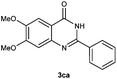
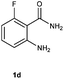
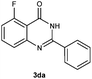
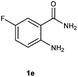
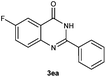
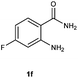
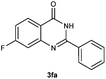
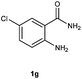
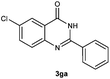
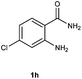
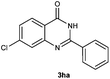
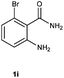
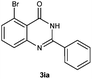
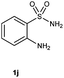
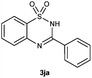
To further expand the scope of reaction, transformations with respect to o-aminobenzamides were then examined. Reactions of o-aminobenzamides bearing one or two electron-donating groups, such as methyl (1b) and dimethoxy (1c), gave the corresponding products 3ba and 3ca in 81% and 78% yields, respectively (Table 3, entries 1 and 2). When o-aminobenzamides bearing an electron-withdrawing group, such as fluoro (1d–f), chloro (1g–h) and bromo (1i), were used as substrates, the desired products 3da–3ia were obtained in 75%–85% yields (Table 3, entries 3–8). The catalytic system was also applied to o-aminobenzenesulfonamide (1j), giving the corresponding product 3ja in only 26% NMR yield (Table 3, entry 9).
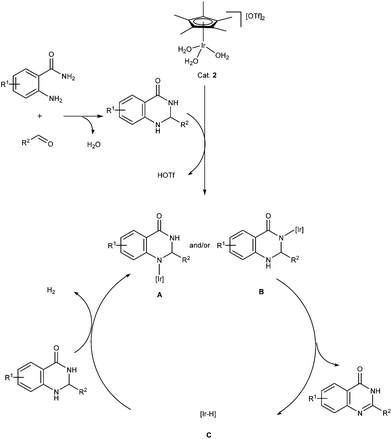
A possible mechanism was proposed to account for the acceptorless dehydrogenative condensation of o-aminobenzamides with aldehydes to quinazolinones in water (Scheme 3). The initial step of the reaction involved the formation of 2,3-dihydroquinazolinones via the condensation between o-aminobenzamides and aldehydes. Furthermore, the reaction of the resulting 2,3-dihydroquinazolinones with Cat. 2 afforded an amido iridium species A and/or B. Accompanied by the β-hydrogen elimination of species A and/or B, the hydrido iridium species C were generated and quinazolinones were released as products.22 Finally, hydrogen gas was liberated and catalytic active species A and/or B were regenerated through the reaction of iridium hydride species C and 2,3-dihydroquinazolinones.
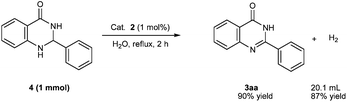
To support the proposed mechanism, the confirmation of the liberation of hydrogen gas in the process of the acceptorless dehydrogenative oxidation of 2-phenyl-2,3-dihydroquinazolin-4(1H)-one (4), which is synthesized via the condensation between 1a and 2a, was first undertaken (Scheme 4). In the presence of Cat. 2 (1 mol%), the reaction was carried out for 2 h in water to give the product 3aa in 90% yield accompanied by the generation of 20.1 mL of gas by water displacement. The collected gas was confirmed to be hydrogen gas by GC analysis and the yield of gas is calculated to be 87%. In addition, a peak (δ −15.6) was observed in the 1H NMR spectrum of the substoichiometric reaction of Cat. 2 with 4 (4 equiv.) in CDCl3 at ambient temperature. It was speculated to be a characteristic signal of [Ir − H] (species C)23 (see the ESI†). These experimental results supported the proposed mechanism shown in Scheme 3.
Conclusion
We have demonstrated a general and efficient method for the synthesis of quinazolinones via the acceptorless dehydrogenative condensation of o-aminobenzamides with aldehydes in water. In the presence of [Cp*Ir(H2O)3][OTf]2, a variety of desirable products were obtained in high yields with high atom economy under environmentally benign conditions. Notably, this research will facilitate the progress of acceptorless dehydrogenative reactions in water catalyzed by water-soluble organometallic complexes.Experimental section
Experimental details
High-resolution mass spectra (HRMS) were obtained on a HPLC-Q-Tof MS(Micro) spectrometer and are reported as m/z (relative intensity). Accurate masses are reported for the deprotonated molecular ion [M − H]−. Proton nuclear magnetic resonance (1H NMR) spectra were recorded at 500 MHz using a 500 spectrometer. Chemical shifts are reported in delta (δ) units, parts per million (ppm) downfield from tetramethylsilane or ppm relative to the center of the singlet at 7.26 ppm for CDCl3 and 2.50 ppm for DMSO-d6. Coupling constant J values are reported in hertz (Hz), and the splitting patterns were designated as follows: s, singlet; d, doublet; t, triplet; m, multiplet; b, broad. Carbon-13 nuclear magnetic resonance (13C NMR) spectra were recorded at 125 MHz using a 500 spectrometer. Chemical shifts are reported in delta (δ) units, ppm relative to the center of the triplet at 77.0 ppm for CDCl3 and 39.5 ppm for DMSO-d6. 13C NMR spectra were routinely run with broadband decoupling.[Cp*Ir(bpy)Cl]Cl (Cat. 1),24 [Cp*Ir(H2O)3][OTf]2 (Cat. 2),25 [Cp*Ir(H2O)3][BF4]2 (Cat. 3),25 [Cp*Ir(H2O)][SO4] (Cat. 4),25 [Cp*Ir(bpy)(H2O)][OTf]2 (Cat. 5)26 and [Cp*Ir(NH3)3][Cl]2 (Cat. 6)27 were synthesized according to previous reports.
General procedure for acceptorless dehydrogenative condensation of o-aminobenzamides with aldehydes to quinazolinones in water catalyzed by a water-soluble iridium complex [Cp*Ir(H2O)3][OTf]2
In a round-bottomed flask with a condenser tube, 2-aminobenzamide 1 (0.5 mmol), aldehyde 2 (0.5 mmol) and water (1 mL) were placed under an air atmosphere, and the reaction mixture was heated under reflux in an oil bath for 2 h. The reaction mixture was further heated under reflux for 1 h when Cat. 2 (0.005 mmol, 1 mol%) was added and the mixture was then cooled to ambient temperature, concentrated in vacuo and purified by flash column chromatography with hexanes/ethyl acetate to afford the corresponding products.Procedure for the hydrogen evolution experiment (Scheme 4)35
4 (1 mmol), Cat. 2 (1 mol%) and H2O (1 mL) were added into a 5 ml thick walled glass vessel fitted with a side arm and a rubber septum. The vessel was previously degassed three times and placed under a N2 atmosphere. The vessel was connected to the gas collection apparatus (standard water displacement apparatus, using a graduated cylinder to determine volume), and the entire system was flushed with N2 for 5 min and allowed to equilibrate for 5 min. The reaction was stirred vigorously at a constant temperature until gas evolution ceased (2 h). The presence of hydrogen in the collected gas was confirmed by GC analysis.The GC analysis was performed on a gas chromatograph with a TCD detector. Injector temperature = 100 °C, column temperature = 50 °C, detector temperature (TCD) = 80 °C, carrier gas = N2, column flow = 20 mL min−1, t = 0.558 min.
The volume of 1 mol of H2 at 283.15 K, 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 810 Pa was calculated according to the van der Waals equation as shown below
810 Pa was calculated according to the van der Waals equation as shown below
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 810 Pa; a = 0.002476 m6 Pa mol−1; b = 0.02661 × 10−3 m3 mol−1; thus, V (H2, 283.15 K, 101
810 Pa; a = 0.002476 m6 Pa mol−1; b = 0.02661 × 10−3 m3 mol−1; thus, V (H2, 283.15 K, 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 810 Pa) = 23.15 L mol−1.
810 Pa) = 23.15 L mol−1.
The collected volume of gas in the experiment above was 20.1 mL, which corresponds to 0.87 mmol of H2.
Procedure for the substoichiometric reaction of [Cp*Ir(H2O)3][OTf]2 with 2-phenyl-2,3-dihydroquinazolin-4(1H)-one
Under an atmosphere of nitrogen, Cat. 2 (6.8 mg, 0.01 mmol), 4 (8.9 mg, 0.04 mmol) and CDCl3 (1.0 mL) were placed in a NMR tube. The NMR tube was put into the NMR probe. After 30 min, 1H NMR analysis was performed. The result is shown in the ESI.†Acknowledgements
Financial support by the National Natural Science Foundation of China (21272115) and the State Key Laboratory of Fine Chemicals (KF1401) is greatly appreciated.Notes and references
- For selected reviews, see: (a) S. B. Mhaske and N. P. Argade, Tetrahedron, 2006, 62, 9787–9826 CrossRef CAS PubMed; (b) I. Khan, A. Ibrar, N. Abbas and A. Saeed, Eur. J. Med. Chem., 2014, 76, 193–244 CrossRef CAS PubMed.
- P. P. Kung, M. D. Casper, K. L. Cook, L. Wilson-Lingard, L. M. Risen, T. A. Vickers, R. Ranken, L. B. Blyn, R. Wyatt, P. D. Cook and D. Ecker, J. Med. Chem., 1999, 42, 4705–4713 CrossRef CAS PubMed.
- N. J. Liverton, D. J. Armstrong, D. A. Claremon, D. C. Remy, J. J. Baldwin, R. J. Lynch, G. Zhang and R. J. Gould, Bioorg. Med. Chem. Lett., 1998, 8, 483–487 CrossRef CAS.
- Z. W. Wang, M. X. Wang, X. Yao, Y. Li, J. Tan, L. Z. Wang, W. T. Qiao, Y. Q. Geng, Y. X. Liu and Q. M. Wang, Eur. J. Med. Chem., 2012, 53, 275–282 CrossRef CAS PubMed.
- S. E. Laszlo, C. S. Quagliato, W. J. Greenlee, A. A. Patchett, R. S. L. Chang, V. J. Lotti, T. B. Chen, S. A. Scheck, K. A. Faust, S. S. Kivlighn, T. S. Schorn, G. J. Zingaro and P. K. S. Siegl, J. Med. Chem., 1993, 36, 3207–3210 CrossRef.
- M. M. Aly, Y. A. Mohamed, K. A. El-Bayouki, W. M. Basyouni and S. Y. Abbas, Eur. J. Med. Chem., 2010, 45, 3365–3373 CrossRef CAS PubMed.
- S. Kobayashi, M. Ueno, R. Suzuki and H. Ishitani, Tetrahedron Lett., 1999, 40, 2175–2178 CrossRef CAS.
- S. L. Cao, Y. P. Feng, Y. Y. Jiang, S. Y. Liu, G. Y. Ding and R. T. Li, Bioorg. Med. Chem. Lett., 2005, 15, 1915–1917 CrossRef CAS PubMed.
- For selected examples, see: (a) X. Liu, H. Fu, Y. Jiang and Y. Zhao, Angew. Chem., Int. Ed., 2009, 48, 348–351 CrossRef CAS PubMed; (b) B. Ma, Y. Wang, J. Peng and Q. Zhu, J. Org. Chem., 2011, 76, 6362–6366 CrossRef CAS PubMed; (c) W. Xu and H. Fu, J. Org. Chem., 2011, 76, 3846–3852 CrossRef CAS PubMed; (d) W. Xu, Y. Jin, H. Liu, H. Jiang and H. Fu, Org. Lett., 2011, 13, 1274–1277 CrossRef CAS PubMed; (e) L. Xu, Y. Jiang and D. Ma, Org. Lett., 2012, 14, 1150–1154 CrossRef CAS PubMed; (f) J. E. R. Sadig, R. Foster, F. Wakenhut and M. C. Willis, J. Org. Chem., 2012, 77, 9473–9486 CrossRef CAS PubMed; (g) Y. F. Wang, F. L. Zhang and S. Chiba, Org. Lett., 2013, 15, 2842–2845 CrossRef CAS PubMed; (h) X. F. Wu, L. He, H. Neumann and M. Beller, Chem. – Eur. J., 2013, 19, 12635–12638 CrossRef CAS PubMed; (i) X. Jiang, T. Tang, J. Wang, Z. Chen, Y. Zhu and S. Ji, J. Org. Chem., 2014, 79, 5082–5087 CrossRef CAS PubMed; (j) Y. F. Wang, F. L. Zhang and S. Chiba, Org. Lett., 2013, 15, 2842–2845 CrossRef CAS PubMed; (k) T. B. Nguyen, J. L. Bescont, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2013, 15, 6218–6221 CrossRef CAS PubMed; (l) X. Yang, G. Cheng, J. Shen, C. Kuai and X. Cui, Org. Chem. Front., 2015, 2, 366–368 RSC.
- (a) T. Hisano, M. Ichikawa, A. Nakagawa and M. Tsuji, Chem. Pharm. Bull., 1975, 23, 1910–1916 CrossRef CAS; (b) C. Balakumar, P. Lamba, D. P. Kishore, B. L. Narayana, K. V. Rao, K. Rajwinder, A. R. Rao, B. Shireesha and B. Narsaiah, Eur. J. Med. Chem., 2010, 45, 4904–4913 CrossRef CAS PubMed; (c) Y. Mitobe, S. Ito, T. Mizutani, T. Nagase, N. Sato and S. Tokita, Bioorg. Med. Chem. Lett., 2009, 19, 4075–4078 CrossRef CAS PubMed; (d) R. J. Abdel-Jalil, W. Voelter and M. Saeed, Tetrahedron Lett., 2004, 45, 3475–3476 CrossRef CAS PubMed; (e) K. Juvale and M. Wiese, Bioorg. Med. Chem. Lett., 2012, 22, 6766–6769 CrossRef CAS PubMed; (f) M. Sharif, J. Opalach, P. Langer, M. Beller and X. Wu, RSC Adv., 2014, 4, 8–17 RSC; (g) R. Cheng, T. Guo, D. Zhang-Negrerie, Y. Du and K. Zhao, Synthesis, 2013, 2998–3006 CAS.
- N. Mulakayala, B. Kandagatla, R. K. Rapolu, P. Rao, C. Mulakayala, C. S. Kumar, J. Iqbal and S. Oruganti, Bioorg. Med. Chem. Lett., 2012, 22, 5063–5066 CrossRef CAS PubMed.
- For a recent review about acceptorless dehydrogenative and related transformations in chemical synthesis, see: B. Gnanaprakasam and D. Milstein, Science, 2013, 341, 1229712 CrossRef PubMed.
- (a) A. Sartbaeva, V. L. Kuznetsov, S. A. Wells and P. P. Edwards, Energy Environ. Sci., 2008, 1, 79–85 RSC; (b) J. D. Holladay, J. Hu, D. L. King and Y. Wang, Catal. Today, 2009, 139, 244–260 CrossRef CAS PubMed; (c) N. Armaroli and V. Balzani, ChemSusChem, 2011, 4, 21–38 CrossRef CAS PubMed.
- (a) K. Fujita, N. Tanino and R. Yamaguchi, Org. Lett., 2007, 9, 109–111 CrossRef CAS PubMed; (b) K. Fujita, T. Yoshida, Y. Imori and R. Yamaguchi, Org. Lett., 2011, 13, 2278–2281 CrossRef CAS PubMed; (c) R. Kawahara, K. Fujita and R. Yamaguchi, Angew. Chem., Int. Ed., 2012, 51, 12790–12794 CrossRef CAS PubMed; (d) S. Musa, I. Shaposhnikov, S. Cohen and D. Gelman, Angew. Chem., Int. Ed., 2011, 50, 3533–3537 CrossRef CAS PubMed; (e) K. T. Tseng, J. W. Kampf and N. K. Szymczak, Organometallics, 2013, 32, 2046–2049 CrossRef CAS; (f) G. Zeng, S. Sakaki, K. Fujita, H. Sano and R. Yamaguchi, ACS Catal., 2014, 4, 1010–1020 CrossRef CAS; (g) H. Song, B. Kang and S. H. Hong, ACS Catal., 2014, 4, 2889–2895 CrossRef CAS; (h) G. Zhang and S. K. Hanson, Org. Lett., 2013, 15, 650–653 CrossRef CAS PubMed.
- (a) R. Yamaguchi, C. Ikeda, T. Takahashi and K. Fujita, J. Am. Chem. Soc., 2009, 131, 8410–8412 CrossRef CAS PubMed; (b) K. Fujita, Y. Tanaka, M. Kobayashi and R. Yamaguchi, J. Am. Chem. Soc., 2014, 136, 4829–4832 CrossRef CAS PubMed; (c) S. Chakraborty, W. W. Brennessel and W. D. Jones, J. Am. Chem. Soc., 2014, 136, 8564–8567 CrossRef CAS PubMed; (d) D. Talwar, A. Gonzalez-de-Castro, H. Y. Li and J. Xiao, Angew. Chem., Int. Ed., 2015, 54, 5223–5227 CrossRef CAS PubMed; (e) Y. Ji, M. W. Chen, L. Shi and Y. G. Zhou, Chin. J. Catal., 2015, 36, 33–39 CrossRef CAS.
- K. T. Tseng, A. M. Rizzi and N. K. Szymczak, J. Am. Chem. Soc., 2013, 135, 16352–16355 CrossRef CAS PubMed.
- S. Kusumoto, M. Akiyama and K. Nozaki, J. Am. Chem. Soc., 2013, 135, 18726–18729 CrossRef CAS PubMed.
- For selected reviews, see: (a) U. M. Lindstrom, Chem. Rev., 2002, 102, 2751–2772 CrossRef PubMed; (b) C. J. Li, Chem. Rev., 2005, 105, 3095–3165 CrossRef CAS PubMed; (c) C. I. Herrerıas, X. Yao, Z. Li and C. J. Li, Chem. Rev., 2007, 107, 2546–2562 CrossRef PubMed; (d) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725–748 CrossRef CAS PubMed; (e) R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302–6337 CrossRef CAS PubMed; (f) M. O. Simon and C. J. Li, Chem. Soc. Rev., 2012, 41, 1415–1427 RSC.
- (a) R. Kawahara, K. Fujita and R. Yamaguchi, J. Am. Chem. Soc., 2012, 134, 3643–3646 CrossRef CAS PubMed; (b) K. Fujita, W. Ito and R. Yamaguchi, ChemCatChem, 2014, 6, 109–112 CrossRef CAS PubMed.
- (a) F. Li, H. Shan, L. Chen, Q. Kang and P. Zou, Chem. Commun., 2012, 48, 603–605 RSC; (b) F. Li, Q. Kang, H. Shan, L. Chen and J. Xie, Eur. J. Org. Chem., 2012, 5085–5092 CrossRef CAS PubMed; (c) F. Li, C. Sun, H. Shan, X. Zou and J. Xie, ChemCatChem, 2013, 5, 1543–1552 CrossRef CAS PubMed; (d) F. Li, P. Qu, J. Ma, X. Zou and C. Sun, ChemCatChem, 2013, 5, 2178–2182 CrossRef CAS PubMed; (e) C. Sun, X. Zou and F. Li, Chem. – Eur. J., 2013, 19, 14030–14033 CrossRef CAS PubMed; (f) J. Ma, N. Wang and F. Li, Asian J. Org. Chem., 2014, 3, 940–947 CrossRef CAS PubMed; (g) N. Wang, X. Zou, J. Ma and F. Li, Chem. Commun., 2014, 50, 8303–8305 RSC; (h) F. Li, C. Sun and N. Wang, J. Org. Chem., 2014, 79, 8031–8039 CrossRef PubMed; (i) F. Li, J. Ma and N. Wang, J. Org. Chem., 2014, 79, 10447–10455 CrossRef CAS PubMed; (j) F. Li, J. Ma, L. Lu, X. Bao and W. Tang, Catal. Sci. Technol., 2015, 5, 1953–1960 RSC; (k) L. Lu, J. Ma, P. Qu and F. Li, Org. Lett., 2015, 17, 2350–2353 CrossRef CAS PubMed.
- (a) C. Sun, P. Qu and F. Li, Catal. Sci. Technol., 2014, 4, 988–996 RSC; (b) P. Qu, C. Sun, J. Ma and F. Li, Adv. Synth. Catal., 2014, 356, 447–459 CrossRef CAS PubMed.
- Fujita, Yamaguchi and co-workers proposed a reverse process from hydrido iridium species to amido iridium species, namely the addition of the hydrido iridium to a C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of cyclic imines occurs to give an amido iridium species, see: K. Fujita, K. Yamamoto and R. Yamaguchi, Org. Lett., 2002, 4, 2691–2694 CrossRef CAS PubMed.
N bond of cyclic imines occurs to give an amido iridium species, see: K. Fujita, K. Yamamoto and R. Yamaguchi, Org. Lett., 2002, 4, 2691–2694 CrossRef CAS PubMed. - Fujita, Yamaguchi and co-workers demonstrated a similar method to verify a characteristic signal of [Ir–H] in the process of iridium-catalyzed dehydrogenative oxidation of alcohols, see ref. 14b.
- R. Ziessel, J. Chem. Soc., Chem. Commun., 1988, 16–17 RSC.
- S. Ogo, N. Makihara and Y. Watanabe, Organometallics, 1999, 18, 5470–5474 CrossRef CAS.
- S. Ogo, N. Makihara, Y. Kaneko and Y. Watanabe, Organometallics, 2001, 20, 4903–4910 CrossRef CAS.
- R. Kawahara, K. Fujita and R. Yamaguchi, J. Am. Chem. Soc., 2010, 132, 15108–15111 CrossRef CAS PubMed.
- M. Hour, L. Huang, S. Kuo, Y. Xia, K. Bastow, Y. Nakanishi, E. Hamel and K. Lee, J. Med. Chem., 2000, 43, 4479–4487 CrossRef CAS PubMed.
- M. Hour, L. Huang, S. Kuo, Y. Xia, K. Bastow, Y. Nakanishi, E. Hamel, K. Lee, A. Couture, H. Cornet and P. Grandclaudon, Synthesis, 1991, 1009–1010 Search PubMed.
- M. M. Heravi, N. Montazeri, M. Rahimzadeh, M. Bakavoli and M. Ghassemzadeh, J. Org. Chem., 2004, 78, 2101–2103 CAS.
- Y. Zhang, M. R. Sheets, E. K. Raja, K. N. Boblak and D. A. Klumpp, J. Am. Chem. Soc., 2011, 133, 8467–8469 CrossRef CAS PubMed.
- X. Zhang, D. Ye, H. Sun, D. Guo, J. Wang, H. Huang, X. Zhang, H. Jiang and H. Liu, Green Chem., 2009, 11, 1881–1888 RSC.
- H. Hikawa, Y. Ino, H. Suzuki and Y. Yokoyama, J. Org. Chem., 2012, 77, 7046–7051 CrossRef CAS PubMed.
- A. Cherepakha, V. O. Kovtunenko, A. Tolmachev and O. Lukin, Tetrahedron, 2011, 67, 6233–6239 CrossRef CAS PubMed.
- B. L. Conley, D. Guess and T. J. Williams, J. Am. Chem. Soc., 2011, 133, 14212–14215 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of the 1H NMR and 13C NMR spectra for all products. See DOI: 10.1039/c5qo00255a |
| This journal is © the Partner Organisations 2015 |