DOI:
10.1039/C5QO00196J
(Research Article)
Org. Chem. Front., 2015,
2, 1346-1351
Copper-catalyzed multi-component synthesis of acrylamidines and benzoimidazoles†
Received
22nd June 2015
, Accepted 3rd August 2015
First published on 4th August 2015
Abstract
Acrylamidines were synthesized via the copper-catalyzed three-component reaction of propargyl acetates, sulfonyl azides and amines, which are readily accessible materials. The synthesized N,N′-bis(aryl)amidines could be converted into 2-styrylbenzoimidazoles by iodobenzene-catalyzed oxidative C–H amination using m-CPBA as a terminal oxidant.
Introduction
Since the breakthrough in the generation of ketenimines via the copper-catalyzed azide–alkyne cycloaddition (CuAAC),1 the chemistry of ketenimines has emerged as a powerful tool in the synthesis of diverse nitrogen containing cyclic and acyclic motifs of biological and pharmaceutical interest.2 Chang,3 our group,4 and others5 have reported a number of elegant studies that involve the transformation of ketenimines generated in situ by the CuAAC route. Most recently, Reddy and coworkers developed a Cu-catalyzed conversion of propargyl acetates to α,β-unsaturated amides via the CuAAC process and 1,3-migration of the acetyl group ([3,3]-sigmatropic rearrangement) (Scheme 1a).6 Similarly, Talukdar's group7 converted propargyl amines to α,β-unsaturated amidines through 1,3-amino group migration of the in situ generated ketenimine intermediate (Scheme 1b). Encouraged by these results and in continuation of our interest in ketenimine chemistry, we herein report a three-component synthesis of acrylamidines via the Cu(I)-catalyzed reaction of readily accessible propargyl acetates, sulfonyl azides and amines, involving the formation of ketenimine and a nucleophilic addition–elimination process (Scheme 1c). The synthesized acrylamidines, i.e. α,β-unsaturated amidines, are an important class of nitrogen-containing compounds due to their synthetic applications.8
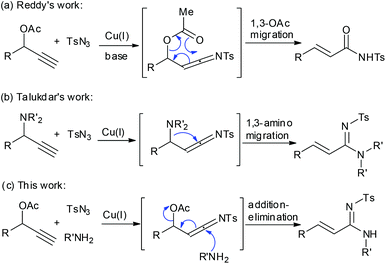 |
| | Scheme 1 Previous work and our design. | |
Results and discussion
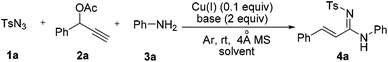
Initially, we selected tosyl azide (1a), 1-phenylprop-2-ynyl acetate (2a) and aniline (1c) as the model substrates to test the feasibility of this transformation. When the reaction was performed in the presence of 10 mol% copper(I) bromide using K2CO3 as the base in acetonitrile at room temperature for 1 hour, α,β-unsaturated amidine 4a was isolated in 60% yield (Table 1, entry 1). The addition of 4 Å MS in the reaction system improved the yield to 76% (Table 1, entry 2). Shortening the reaction time led to a decrease in the yield of 4a (Table 1, entry 3), while the formation of 4a was not favoured when the reaction was performed for an additional reaction time (Table 1, entry 4). Increasing the amount of aniline improved the yield to 85% (Table 1, entry 5). Changing the catalyst (Table 1, entries 6–8), base (Table 1, entries 9–11) or solvent (Table 1, entries 12–15) led to decreases in the yield of 4a. The reaction proceeded at 0 °C for 3 hours and furnished 4a with only 45% yield (Table 1, entry 16). Increasing the reaction temperature to 50 °C also led to a decrease in the yield (Table 1, entry 17).
Table 1 Screening of the reaction conditionsa
|
|
| Entry |
Catalyst |
Base |
3a (equiv.) |
Solvent |
Time (h)c |
Yieldd (%) |
|
Reaction conditions: 1a (0.5 mmol), 2a (0.5 mmol), Cu salt (0.05 mmol), 4 Å MS (100 mg), base (1.0 mmol), solvent (2 mL), room temperature.
Without 4 Å MS, base (2.0 mmol).
The reaction time was determined by TLC.
Isolated yield.
The reaction was carried out at 0 °C.
The reaction was carried out at 50 °C.
|
| 1 |
CuBr |
K2CO3 |
1 |
MeCN |
1 |
60b |
| 2 |
CuBr |
K2CO3 |
1 |
MeCN |
1 |
76 |
| 3 |
CuBr |
K2CO3 |
1 |
MeCN |
0.5 |
57 |
| 4 |
CuBr |
K2CO3 |
1 |
MeCN |
1.5 |
75 |
| 5 |
CuBr |
K2CO3 |
2 |
MeCN |
1 |
85 |
| 6 |
CuI |
K2CO3 |
2 |
MeCN |
1 |
71 |
| 7 |
CuCl |
K2CO3 |
2 |
MeCN |
1 |
38 |
| 8 |
CuOTf |
K2CO3 |
2 |
MeCN |
1 |
56 |
| 9 |
CuBr |
Cs2CO3 |
2 |
MeCN |
1 |
40 |
| 10 |
CuBr |
Pyridine |
2 |
MeCN |
1 |
46 |
| 11 |
CuBr |
NEt3 |
2 |
MeCN |
1 |
42 |
| 12 |
CuBr |
K2CO3 |
2 |
THF |
1 |
42 |
| 13 |
CuBr |
K2CO3 |
2 |
DMF |
1 |
47 |
| 14 |
CuBr |
K2CO3 |
2 |
PhMe |
1 |
23 |
| 15 |
CuBr |
K2CO3 |
2 |
DCE |
1 |
46 |
| 16 |
CuBr |
K2CO3 |
2 |
MeCN |
3 |
45e |
| 17 |
CuBr |
K2CO3 |
2 |
MeCN |
1 |
49f |
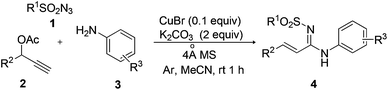
To establish the scope of the methodology, the optimized conditions were applied to a wide range of substrates. As shown in Table 2, p-methylbenzenesulfonyl, phenylsulfonyl and p-methoxybenzenesulfonyl azides (1a–1c) gave the desired products 4a–4c with satisfactory yields (Table 2, entries 1–3), whereas naphthalen-2-ylsulfonyl, 4-(acetylamino)-benzenesulfonyl, 4-nitrobenzenesulfonyl, 4-fluorobenzene-sulfonyl and methylsulfonyl azides (1d–1h) yielded the corresponding products 4d–4h in much lower yields (Table 2, entries 4–8). When the R2 group on propargyl acetates 2 was 4-methoxyphenyl (2b), 4-methylphenyl (2c), styryl (2d), and 2-furanyl (2e), the corresponding products 4i–4l were isolated in 54%–67% yields (Table 2, entries 9–12). In the case where R2 was 4-nitrophenyl (2f), a lower yield (43%) of 4m was obtained (Table 2, entry 13). The substituted anilines were also examined for their reaction with 1a and 2a under the standard conditions. 4-Methylaniline (3b), 4-methoxyaniline (3c), and 2-methylaniline (3d) gave the corresponding products 4n–4p in good to excellent yields (Table 2, entries 14–16), whereas 4-chloroaniline (3e) furnished the desired product 4q in poor yield due to the electron-withdrawing properties of the chlorine atom (Table 2, entry 17).
Table 2 Substrate scope of azidesa
|
|
| Entry |
1 (R1) |
2 (R2) |
3 (R3) |
4/yieldb (%) |
|
Reaction conditions: 1 (0.5 mmol), 2a (0.5 mmol), 3a (1 mmol), CuBr (0.05 mmol), 4 Å MS (100 mg), acetonitrile (2 mL), Ar, room temperature, 1 h.
Product yields are given for compounds isolated after purification from a silica gel column.
|
| 1 |
1a (4-MeC6H4) |
2a
|
3a (H) |
4a/88 |
| 2 |
1b (Ph) |
2a
|
3a
|
4b/67 |
| 3 |
1c (4-MeOC6H4) |
2a
|
3a
|
4c/97 |
| 4 |
1d (2-naphthalenyl) |
2a
|
3a
|
4d/34 |
| 5 |
1e (4-AcNHC6H4) |
2a
|
3a
|
4e/43 |
| 6 |
1f (4-NO2C6H4) |
2a
|
3a
|
4f/26 |
| 7 |
1g (4-FC6H4) |
2a
|
3a
|
4g/39 |
| 8 |
1h (Me) |
2a
|
3a
|
4h/39 |
| 9 |
1a
|
2b (4-MeOC6H4) |
3a
|
4i/60 |
| 10 |
1a
|
2c (4-MeC6H4) |
3a
|
4j/67 |
| 11 |
1a
|
2d (PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) CH) |
3a
|
4k/63 |
| 12 |
1a
|
2e (2-furanyl) |
3a
|
4l/54 |
| 13 |
1a
|
2f (4-NO2C6H4) |
3a
|
4m/43 |
| 14 |
1a
|
2a
|
3b (4-Me) |
4n/95 |
| 15 |
1a
|
2a
|
3c (4-MeO) |
4o/88 |
| 16 |
1a
|
2a
|
3d (2-Me) |
4p/84 |
| 17 |
1a
|
2a
|
3e (4-Cl) |
4q/20 |
As a synthetic application of our methodology, we converted the synthesized N,N′-bis(aryl)amidines 4a–4h (Table 3, entries 1–8) and 4n–4p (Table 3, entries 9–11) into 2-styrylbenzoimidazoles 5a–5k by iodobenzene-catalyzed oxidative C–H amination in the presence of m-CPBA as a terminal oxidant at room temperature (Table 3).9 The reaction is general although the target products were obtained in lower yields in most cases. Taking into consideration the great importance of benzoimidazoles in medicinal chemistry10 and organic materials,11 our two-step approach provided a new strategy to construct 2-styrylbenzoimidazoles.
Table 3 Preparation of benzoimidazolesa
|

|
| Entry |
4 (R1/R2/R3) |
5/yieldb (%) |
|
Reaction conditions: 4 (0.2 mmol), PhI (5 μL), m-CPBA (0.3 mmol), HFIP (0.4 mL), room temperature, 8 h.
Product yields are given for compounds isolated after purification from a silica gel column.
|
| 1 |
4a (4-MeC6H4/Ph/H) |
5a/64 |
| 2 |
4b (Ph/Ph/H) |
5b/44 |
| 3 |
4c (4-MeOC6H4/Ph/H) |
5c/45 |
| 4 |
4d (2-naphthalenyl/Ph/H) |
5d/44 |
| 5 |
4h (Me/Ph/H) |
5e/25 |
| 6 |
4f (4-NO2C6H4/Ph/H) |
5f/22 |
| 7 |
4g (4-FC6H4/Ph/H) |
5g/41 |
| 8 |
4e (4-AcNHC6H4/Ph/H) |
5h/29 |
| 9 |
4o (4-MeC6H4/Ph/4-MeO) |
5i/30 |
| 10 |
4n (4-MeC6H4/Ph/4-Me) |
5j/36 |
| 11 |
4p (4-MeC6H4/Ph/2-Me) |
5k/30 |
Conclusions
We developed a copper-catalyzed three-component synthesis of α,β-unsaturated amidines from propargyl acetates, sulfonyl azides and amines, which are readily accessible materials. The reaction proceeded through a cascade process involving ketenimine formation via copper-catalyzed alkyne–azide cycloaddition, nucleophilic addition of amine to ketenimine, and elimination of acetate. Furthermore, the synthesized N,N′-bis(aryl)amidines could be converted into 2-styrylbenzoimidazoles by iodobenzene-catalyzed oxidative C–H amination using m-CPBA as a terminal oxidant.
Experimental section
General methods
Infrared spectra were obtained on a FTIR spectrometer. 1H NMR spectra were recorded on a 400 MHz spectrometer, referred to the internal solvent signals (0 for TMS in CDCl3 or 2.5 for the residue of DMSO). The following abbreviations were used to describe peak patterns where appropriate: b = broad, s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet. Coupling constants were reported in Hertz (Hz). 13C NMR spectra were recorded on a 100 MHz spectrometer, referred to the internal solvent signals (77.27 for CDCl3 or 40.0 for DMSO-d6). High resolution mass spectra (HRMS) were recorded on an electron ionization time-of-flight (EI-TOF) mass spectrometer. Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDITOF-MS) was performed with a Bruker's ultrafleXtreme. Melting points were uncorrected and measured with a micro melting point apparatus.
General procedure for the synthesis of (E)-acrylimidamide 4
To a solution of K2CO3 (1.0 mmol), 4 Å MS (100 mg) and CuBr (0.05 mmol) in MeCN (0.5 mL) protected by argon was added sulfonyl azide 1 (0.5 mmol) in MeCN (0.5 mL), 2-yn-1-yl acetate 2 (0.5 mmol) in MeCN (0.5 mL) and aniline 3 (0.5 mmol) in MeCN (0.5 mL) in turns via a syringe, and the mixture was reacted at room temperature for 1 h. After filtration, the reaction mixture was diluted with CH2Cl2 (10 mL), washed with water and brine, dried over anhydrous Na2SO4, and evaporated in a vacuum. The residue was subjected to silica gel column chromatography with EA/Pet (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) as the eluent to give pure product 4.
3, v/v) as the eluent to give pure product 4.
(E)-N-Phenyl-N′-tosylcinnamimidamide (4a).
A yellow powder (166 mg, 88%); m.p. 170.0–171.6 °C; 1H NMR (400 MHz, CDCl3) δ 10.14 (b, 1H), 7.93 (d, J = 8.4 Hz, 2H), 7.85 (d, J = 15.6 Hz, 1H), 7.42 (dd, J1 = 7.6 Hz, J2 = 7.2 Hz, 2H), 7.37–7.27 (m, 8H), 7.19 (d, J = 7.6, 2H), 6.51 (d, J = 11.2 Hz, 1H), 2.43 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.2, 144.7, 143.1, 140.0, 136.6, 134.7, 130.7, 129.8, 129.7, 129.1, 128.5, 127.8, 126.7, 117.8, 21.8; IR (neat) ν 3298, 3060, 3021, 1635, 1561, 1519, 1445, 1389, 1350, 1276, 1142, 1089, 758, 691 cm−1; HRMS (EI) calcd for chemical formula: C22H20N2O2S, 376.1245; found, 376.1241.
(E)-N-Phenyl-N′-(phenylsulfonyl)cinnamimidamide (4b).
A yellow powder (121 mg, 67%); m.p. 164.1–165.3 °C; 1H NMR (400 MHz, CDCl3) δ 10.16 (b, 1H), 8.05 (d, J = 8.4 Hz, 2H), 7.87 (d, J = 15.2 Hz, 1H), 7.59–7.50 (m, 3H), 7.43 (dd, J1 = 8.0 Hz, J2 = 7.2 Hz, 2H), 7.37–7.29 (m, 6H), 7.19 (d, J = 7.2 Hz, 2H), 6.51 (b, 1H); 13C NMR (100 MHz, CDCl3) δ 160.4, 142.8, 136.5, 134.6, 132.5, 130.8, 129.9, 129.1, 129.1, 128.6, 127.9, 126.6, 126.1, 117.7; IR (neat) ν 3299, 33048, 3026, 1635, 1561, 1518, 1445, 1397, 1346, 1277, 1143, 1089, 754, 689 cm−1; HRMS (EI) calcd for C21H18N2O2S, 362.1089; found, 362.1084.
(E)-N′-((4-Methoxyphenyl)sulfonyl)-N-phenyl cinnamimidamide (4c).
A yellow powder (191 mg, 97%); m.p. 146.2–47.5 °C; 1H NMR (400 MHz, CDCl3) δ 10.11 (b, 1H), 7.98 (d, J = 8.8 Hz, 2H), 7.83 (d, J = 15.2 Hz, 1H), 7.42 (dd, J1 = 7.6 Hz, J2 = 7.2 Hz, 2H), 7.36–7.28 (m, 6H), 7.19 (d, J = 6.8 Hz, 2H), 6.98 (d, J = 9.2 Hz, 2H), 6.52 (b, 1H), 3.86 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.8, 160.0, 144.5, 136.7, 134.8, 134.7, 130.7, 129.8, 129.1, 128.7, 128.5, 127.8, 126.0, 117.8, 114.2, 55.8; IR (neat) ν 3293, 3060, 2944, 2837, 1650, 1596, 1562, 1519, 1497, 1444, 1257, 1141, 1090, 1026, 805, 759, 692 cm−1; HRMS (EI) calcd for C22H20N2O3S, 392.1195; found, 392.1190.
(E)-N′-(Naphthalen-2-ylsulfonyl)-N-phenylcinnamimidamide (4d).
A light yellow powder (71 mg, 34%); m.p. 167.0–168.2 °C; 1H NMR (400 MHz, CDCl3) δ 10.23 (b, 1H), 8.59 (s, 1H), 8.05 (dd, J1 = 8.4 Hz, J2 = 1.6 Hz, 1H), 7.98 (d, J = 8.4 Hz, 2H), 7.92–7.85 (m, 2H), 7.64–7.57 (m, 2H), 7.43 (dd, J1 = J2 = 7.6 Hz, 2H), 7.36–7.28 (m, 6H), 7.21 (d, J = 7.2 Hz, 2H), 6.53 (b, 1H); 13C NMR (100 MHz, CDCl3) δ 160.4, 139.7, 136.5, 135.0, 134.6, 132.4, 130.8, 129.9, 129.6, 129.4, 129.1, 128.7, 128.6, 128.1, 127.9, 127.5, 127.3, 126.1, 122.7, 117.7; IR (neat) ν 3289, 3054, 1635, 1563, 1520, 1445, 1382, 1348, 1278, 1143, 1124, 1073, 756, 690 cm−1; HRMS (EI) calcd for C25H20N2O2S, 412.1245; found, 412.1252.
N-(4-((E)-N-((E)-3-Phenyl-1-(phenylamino)allylidene)sulfamoyl)phenyl)acetamide (4e).
A yellow powder (90 mg, 43%); m.p. 172.2–173.5 °C; 1H NMR (400 MHz, CDCl3) δ 10.10 (b, 1H), 8.01 (s, 1H), 7.93 (d, J = 8.8 Hz, 2H), 7.82 (d, J = 14.8 Hz, 1H), 7.67 (d, J = 7.6 Hz, 2H), 7.42 (dd, J1 = 7.2 Hz, J2 = 8.0 Hz, 2H), 7.36–7.28 (m, 6H), 7. 17 (d, J = 5.2 Hz, 2H), 6.50 (b, 1H), 2.20 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.2, 160.4, 145.0, 142.0, 137.4, 136.4, 134.5, 130.8, 129.9, 129.1, 128.6, 128.0, 127.7, 126.1, 119.6, 117.6, 24.9; IR (neat) ν 3315, 3048, 2920, 1676, 1635, 1593, 1519, 1446, 1400, 1315, 1262, 1141, 1091, 754 cm−1; HRMS (EI) calcd for C23H21N3O3S, 419.1304; found, 419.1305.
(E)-N′-((4-Nitrophenyl)sulfonyl)-N-phenylcinnamimidamide (4f).
A white powder (53 mg, 26%); m.p. 148.8–149.6 °C; 1H NMR (400 MHz, CDCl3) δ 10.15 (b, 1H), 8.37 (d, J = 8.8 Hz, 2H), 8.23 (d, J = 8.8 Hz, 2H), 7.87 (d, J = 13.6 Hz, 1H), 7.47 (dd, J1 = J2 = 7.6 Hz, 2H), 7.42–7.32 (m, 6H), 7.22 (d, J = 7.2 Hz, 2H), 6.52 (b, 1H); 13C NMR (100 MHz, CDCl3) δ 161.1, 150.0, 148.5, 146.1, 136.1, 134.3, 131.2, 130.0, 129.2, 128.7, 128.4, 127.9, 126.2, 124.4, 117.0; IR (neat) ν 3319, 3024, 1635, 1521, 1447, 1348, 1262, 1145, 1092, 745, 680 cm−1; MALDITOF-MS (DHB) calcd for C21H18N3O4S [M + H]+, 408.102; found, 408.101.
(E)-N′-((4-Fluorophenyl)sulfonyl)-N-phenylcinnamimidamide (4g).
A yellow powder (74 mg, 39%); m.p. 162.6–163.5 °C; 1H NMR (400 MHz, CDCl3) δ 10.12 (b, 1H), 8.07–8.04 (m, 2H), 7.85 (d, J = 14.4 Hz, 1H), 7.44 (dd, J1 = 7.2 Hz, J2 = 8.0 Hz, 2H), 7.36–7.30 (m, 6H), 7.21–7.17 (m, 4H), 6.53 (b, 1H); 13C NMR (100 MHz, CDCl3) δ 165.1 (d, JC–F = 252 Hz), 160.4, 145.1, 139.0 (d, JC–F = 3.1 Hz), 136.4, 134.6, 130.9, 129.9, 129.3 (d, JC–F = 9.2 Hz), 129.2, 128.6, 128.0, 126.1, 117.6, 116.2 (d, JC–F = 22.4 Hz); IR (neat) ν 3424, 2997, 2911, 1636, 1518, 1494, 1445, 1281, 1146, 1092, 1028, 953, 762, 696 cm−1; HRMS (EI) calcd for C21H17FN2O2S, 380.0995; found, 380.0994.
(E)-N′-(Methylsulfonyl)-N-phenylcinnamimidamide (4h).
A yellow oil (59 mg, 39%); 1H NMR (400 MHz, CDCl3) δ 9.86 (b, 1H), 7.85 (d, J = 11.2 Hz, 1H), 7.45–7.39 (m, 4H), 7.38–7.31 (m, 4H), 7.22 (d, J = 5.2 Hz, 2H), 6.55 (b, 1H), 3.16 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.2, 144.3, 136.5, 134.6, 130.7, 129.8, 129.2, 128.5, 127.8, 125.9, 117.8, 42.9; IR (neat) ν 3290, 3057, 3024, 2929, 1637, 1577, 1525, 1445, 1275, 1124, 1028, 969, 791, 758, 692 cm−1; HRMS (EI) calcd for C16H16N2O2S, 300.0932; found, 300.0933
(1E,2E)-3-(4-Methoxyphenyl)-N-phenyl-N′-tosylacrylimidamide (4i).
A yellow powder (123 mg, 60%); m.p. 174.8–176.2 °C; 1H NMR (400 MHz, CDCl3) δ 10.08 (b, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.82 (d, J = 15.2 Hz, 1H), 7.42 (dd, J1 = J2 = 7.6 Hz, 2H), 7.36–7.30 (m, 5H), 7.18 (d, J = 7.6 Hz, 2H), 6.83 (d, J = 8.8 Hz, 2H), 6.38 (d, J = 14.0 Hz, 1H), 3.80 (s, 3H), 2.42 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 161.8, 160.6, 143.0, 140.1, 136.8, 130.4, 129.8, 129.6, 127.7, 127.5, 126.6, 126.1, 115.1, 114.6, 55.6, 21.8; IR (neat) ν 3304, 3060, 2965, 2929, 2834, 1633, 1601, 1558, 1510, 1444, 1254, 1174, 1141, 1090, 1029, 825, 757, 691 cm−1; HRMS (EI) calcd for C23H22N2O3S, 406.1351; found, 406.1350.
(1E,2E)-N-Phenyl-3-(p-tolyl)-N′-tosylacrylimidamide (4j).
A yellow powder (131 mg, 67%); m.p. 166.7–168.0 °C; 1H NMR (400 MHz, CDCl3) δ 10.11 (b, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.83 (d, J = 15.2 Hz, 1H), 7.42 (dd, J1 = J2 = 7.6 Hz, 2H), 7.35 (d, J = 7.2 Hz, 1H), 7.31 (d, J = 8.0 Hz, 2H), 7.25 (d, J = 6.4 Hz, 2H), 7.18 (d, J = 7.6 Hz, 2H), 7.12 (d, J = 6.4 Hz, 2H), 6.47 (b, 1H), 2.42 (s, 3H), 2.33 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.5, 144.9, 143.1, 141.3, 140.0, 136.7, 132.0, 129.9, 129.8, 129.7, 128.6, 127.8, 126.7, 126.1, 116.6, 21.8, 21.7; IR (neat) ν 3300 3054, 2920, 1634, 1599, 1561, 1520, 1444, 1275, 1142, 1089, 810, 757 cm−1; HRMS (EI) calcd for C23H22N2O2S, 390.1402; found, 390.1402.
(1E,2E,4E)-N,5-Diphenyl-N′-tosylpenta-2,4-dienimidamide (4k).
A yellow powder (127 mg, 63%); m.p. 170.6–172.2 °C; 1H NMR (400 MHz, CDCl3) δ 10.03 (b, 1H), 7.91 (d, J = 8.0 Hz, 2H), 7.63 (dd, J1 = 12.4 Hz, J2 = 13.2 Hz, 1H), 7.43–7.38 (m, 4H), 7.35–7.28 (m, 6H), 7.16 (d, J = 7.6, 2H), 6.89 (d, J = 15.6 Hz, 1H), 6.73 (dd, J1 = 15.6 Hz, J2 = 11.2 Hz, 1H), 6.07 (b, 1H), 2.42 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.3, 145.1, 143.0, 141.6, 140.1, 136.7, 136.1, 130.0, 129.6, 129.5, 129.0, 127.8, 127.5, 126.6, 126.2, 120.8, 21.8; IR (neat) ν 3297, 3059, 3021, 1622, 1557, 1444, 1386, 1352, 1276, 1142, 1090, 996, 748, 691 cm−1; HRMS (EI) calcd for C24H22N2O2S, 402.1402; found, 402.1406.
(1E,2E)-3-(Furan-2-yl)-N-phenyl-N′-tosylacrylimidamide (4l).
A yellow powder (98 mg, 54%); m.p. 120.5–121.4 °C; 1H NMR (400 MHz, CDCl3) δ 10.09 (b, 1H), 7.91 (d, J = 8.0 Hz, 2H), 7.64 (d, J = 16.8 Hz, 1H), 7.45–7.29 (m, 6H), 7.17 (d, J = 7.6 Hz, 2H), 6.58 (d, J = 3.6 Hz, 1H), 6.43–6.37 (m, 2H), 2.42 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.2, 151.3, 145.4, 143.1, 140.1, 136.6, 131.1, 129.8, 129.6, 127.9, 126.6, 126.2, 116.3, 115.1, 112.8, 21.8; IR (neat) ν 3286, 2956, 2923, 2851, 1733, 1635, 1571, 1515, 1444, 1378, 1276, 1142, 1090, 1017, 744 cm−1; HRMS (EI) calcd for C20H18N2O3S, 366.1038; found, 366.1039.
(1E,2E)-3-(4-Nitrophenyl)-N-phenyl-N′-tosylacrylimidamide (4m).
A yellow powder (91 mg, 43%); m.p. 222.4–223.2 °C; 1H NMR (400 MHz, CDCl3) δ 10.22 (b, 1H), 8.17 (d, J = 8.8 Hz, 2H), 7.93 (d, J = 8.4 Hz, 2H), 7.84 (d, J = 14.4 Hz, 1H), 7.50–7.44 (m, 4H), 7.39 (dd, J1 = 7.6 Hz, J2 = 6.8 Hz, 1H), 7.33 (d, J = 8.4 Hz, 2H), 7.19 (d, J = 6.8, 2H), 6.61 (d, J = 14.4 Hz, 1H), 2.44 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.0, 148.6, 143.5, 141.4, 140.7, 139.5, 136.3, 130.0, 129.8, 129.0, 128.3, 126.7, 126.1, 124.4, 122.1, 21.8; IR (neat) ν 3289, 1599, 1560, 1519, 1445, 1343, 1276, 1143, 1089, 758, 692 cm−1; HRMS (EI) calcd for C22H19N3O4S, 421.1096; found, 421.1088.
(E)-N-(p-Tolyl)-N′-tosylcinnamimidamide (4n).
A yellow powder (185 mg, 95%); m.p. 169.4–170.7 °C; 1H NMR (400 MHz, CDCl3) δ 10.08 (b, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.85 (d, J = 15.2 Hz, 1H), 7.37–7.30 (m, 7H), 7.21 (d, J = 8.4 Hz, 2H), 7.05 (d, J = 8.4 Hz, 2H), 6.49 (d, J = 14.0 Hz, 1H), 2.42 (s, 3H), 2.39 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.3, 144.7, 143.1, 140.0, 134.7, 133.9, 130.6, 130.4, 129.6, 129.1, 128.5, 127.3, 126.6, 126.0, 117.8, 21.8, 21.3; IR (neat) ν 3298, 3021, 2920, 2858, 1745, 1640, 1557, 1510, 1449, 1407, 1275, 1139, 1088, 815, 760, 694 cm−1; HRMS (EI) calcd for C23H22N2O2S, 390.1402; found, 390.1405.
(E)-N-(4-Methoxyphenyl)-N′-tosylcinnamimidamide (4o).
A light yellow powder (178 mg, 88%); m.p. 150.3–151.5 °C; 1H NMR (400 MHz, CDCl3) δ 10.01 (b, 1H), 7.93 (d, J = 8.0 Hz, 2H), 7.85 (d, J = 15.2 Hz, 1H), 7.36–7.30 (m, 7H), 7.09 (d, J = 8.0 Hz, 2H), 6.93 (d, J = 8.8 Hz, 2H), 6.43 (d, J = 15.2 Hz, 1H), 3.85 (s, 3H), 2.43 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.6, 159.3, 144.7, 143.1, 140.1, 134.7, 130.6, 129.6, 129.2, 129.1, 128.5, 127.9, 126.6, 117.6, 115.0, 55.8, 21.8; IR (neat) ν 3298, 3021, 2926, 2828, 1636, 1557, 1509, 1449, 1250, 1138, 1088, 1031, 808, 760, 694 cm−1; HRMS (EI) calcd for C23H22N2O3S, 406.1351; found, 406.1353.
(E)-N-(o-Tolyl)-N′-tosylcinnamimidamide (4p).
A light yellow powder (163 mg, 84%); m.p. 113.6–114.8 °C; 1H NMR (400 MHz, CDCl3) δ 9.96 (b, 1H), 7.94 (d, J = 8.0 Hz, 2H), 7.90 (d, J = 15.2 Hz, 1H), 7.35–7.29 (m, 9H), 7.27–7.23 (m, 1H), 7.12 (d, J = 7.2 Hz, 1H), 6.33 (d, J = 15.2 Hz, 1H), 2.43 (s, 3H), 2.24 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.6, 145.0, 143.1, 140.0, 135.2, 134.8, 134.7, 131.6, 130.8, 129.7, 129.1, 128.6, 128.6, 127.6, 127.3, 126.7, 117.3, 21.8, 18.2; IR (neat) ν 3271, 3060, 3021, 1637, 1557, 1449, 1386, 1275, 1138, 1084, 758, 694 cm−1; HRMS (EI) calcd for C23H22N2O2S, 390.1402; found, 390.1408.
(E)-N-(m-Tolyl)-N′-tosylcinnamimidamide (4q).
A light yellow powder (98 mg, 84%); m.p. 186.7–187.7 °C; 1H NMR (400 MHz, CDCl3) δ 10.11 (b, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.85 (d, J = 14.8 Hz, 1H), 7.37–7.28 (m, 8H), 7.16 (d, J = 7.6 Hz, 1H), 7.00–6.96 (m, 2H), 6.52 (d, J = 13.6 Hz, 1H), 2.43 (s, 3H), 2.38 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.2, 144.6, 143.1, 140.1, 140.0, 136.5, 134.7, 130.7, 129.7, 129.6, 129.1, 128.7, 128.6, 126.7, 123.2, 117.9, 21.8, 21.6; IR (neat) ν 3291, 2920, 1637, 1563, 1525, 1486, 1447, 1276, 1143, 1087, 811, 781, 760 cm−1; HRMS (EI) calcd for C23H22N2O2S, 390.1402; found, 390.1399.
(E)-N-(4-Chlorophenyl)-N′-tosylcinnamimidamide (4r).
A white powder (42 mg, 20%); m.p. 193.1–193.8 °C; 1H NMR (400 MHz, CDCl3) δ 10.06 (b, 1H), 7.91 (d, J = 8.0 Hz, 2H), 7.83 (d, J = 14.4 Hz, 1H), 7.40–7.30 (m, 9H), 7.14 (d, J = 7.6, 2H), 6.48 (b, 1H), 2.43 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.0, 143.3, 139.8, 135.2, 134.5, 130.9, 130.0, 129.7, 129.2, 128.6, 127.2, 126.7, 117.4, 100.2, 21.8; IR (neat) ν 3295, 2975, 1635, 1513, 1491, 1401, 1273, 1143, 1088, 688 cm−1; HRMS (EI) calcd for C22H19ClN2O2S, 410.0856; found, 410.0851.
General procedure for the synthesis of (E)-2-vinyl-1-sulfonyl-1H-benzo[d]imidazole (5)
A mixture of (E)-acrylimidamide 4 (0.2 mmol), PhI (5.0 μL) and m-CPBA (0.3 mmol) in HFIP (0.4 mL) was stirred at room temperature for 8 h, and then subjected to silica gel column chromatography with EA/Pet (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) as the eluent to give pure product 5.
4, v/v) as the eluent to give pure product 5.
(E)-2-Styryl-1-tosyl-1H-benzo[d]imidazole (5a).
A fresh solid (48 mg, 64%); m.p. 152.1–153.5 °C; 1H NMR (400 MHz, CDCl3) δ 8.11–8.06 (m, 1H), 7.92 (s, 2H), 7.78 (d, J = 8.4 Hz, 2H), 7.70–7.66 (m, 3H), 7.46–7.33 (m, 5H), 7.22 (d, J = 8.4 Hz, 2H), 2.34 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 151.4, 146.2, 142.9, 140.1, 136.0, 135.5, 133.4, 130.4, 129.9, 129.2, 128.0, 127.1, 125.5, 125.3, 120.1, 114.6, 114.2, 21.9; IR (neat) ν 3099, 3050, 3018, 2923, 2852, 1625, 1396, 1512, 1448, 1378, 1344, 1200, 1167, 1121, 1089, 1050, 811, 764, 670 cm−1; HRMS (EI) calcd for C22H18N2O2S, 374.1089; found, 374.1086.
(E)-1-(Phenylsulfonyl)-2-styryl-1H-benzo[d]imidazole (5b).
A yellow oil (32 mg, 44%); 1H NMR (400 MHz, CDCl3) δ 8.11–8.07 (m, 1H), 7.93–7.89 (m, 4H), 7.72–7.66 (m, 3H), 7.57 (dd, J1 = J2 = 7.6 Hz, 1H), 7.47–7.43 (m, 4H), 7.41–7.34 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 151.4, 142.9, 140.2, 138.5, 136.0, 134.8, 133.4, 129.9, 129.8, 129.2, 128.0, 127.0, 125.6, 125.4, 120.2, 114.5, 114.2; IR (neat) ν 3057, 3021, 1625, 1513, 1379, 1344, 1201, 1167, 1088, 1049, 749, 723, 684, 649 cm−1; HRMS (EI) calcd for C21H16N2O2S, 360.0932; found, 360.0931.
(E)-1-((4-Methoxyphenyl)sulfonyl)-2-styryl-1H-benzo[d]imidazole (5c).
A pink oil (36 mg, 45%); 1H NMR (400 MHz, CDCl3) δ 8.09–8.07 (m, 1H), 7.93 (d, J = 2.4 Hz, 2H), 7.84 (d, J = 9.2 Hz, 2H), 7.70–7.66 (m, 3H), 7.46–7.33 (m, 5H), 6.87 (d, J = 8.8 Hz, 2H), 3.78 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 164.6, 151.4, 142.9, 140.0, 136.0, 133.4, 129.9, 129.8, 129.4, 129.2, 128.0, 125.5, 125.3, 120.1, 115.0, 114.7, 114.2, 56.0; IR (neat) ν 3060, 3025, 1593, 1497, 1448, 1377, 1343, 1266, 1201, 1164, 1090, 1050, 745, 674 cm−1; HRMS (EI) calcd for C22H18N2O3S, 390.1038; found, 390.1035.
(E)-1-(Naphthalen-2-ylsulfonyl)-2-styryl-1H-benzo[d]imidazole (5d).
A yellow oil (36 mg, 44%); 1H NMR (400 MHz, CDCl3) δ 8.55 (d, J = 1.6 Hz, 1H), 8.16–8.13 (m, 1H), 8.04–7.89 (m, 3H), 7.84 (dd, J1 = 7.2, J2 = 10.4 Hz, 2H), 7.46 (dd, J1 = 8.8, J2 = 2.0 Hz, 1H), 7.69–7.56 (m, 5H), 7.48–7.34 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 151.4, 142.9, 140.3, 136.0, 135.7, 135.2, 133.4, 132.0, 130.4, 130.1, 129.9, 129.8, 129.2, 129.1, 128.3, 128.2, 128.0, 125.6, 125.4, 121.3, 120.2, 114.7, 114.2; IR (neat) ν 3057, 3027, 2920, 2852, 1625, 1512, 1448, 1380, 1345, 1231, 1199, 1167, 1072, 1050, 857, 811, 746, 662 cm−1; HRMS (EI) calcd for C25H18N2O2S, 410.1089; found, 410.1086.
(E)-1-(Methylsulfonyl)-2-styryl-1H-benzo[d]imidazole (5e).
A yellow solid (15 mg, 25%); m.p. 192.4–193.4 °C; 1H NMR (400 MHz, CDCl3) δ 8.03 (d, J = 16.0 Hz, 1H), 7.95–7.93 (m, 1H), 7.80–7.74 (m, 2H), 7.66–7.64 (m, 2H), 7.45–7.36 (m, 5H), 3.24 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 151.2, 142.9, 140.8, 135.8, 133.3, 130.0, 129.2, 128.1, 125.8, 125.5, 120.4, 113.9, 113.7, 42.6; IR (neat) ν 2923, 2846, 1626, 1513, 1172, 1233, 1200, 1163, 1144, 1054, 964, 764, 739, 696 cm−1; HRMS (EI) calcd for C16H14N2O2S, 298.0776; found, 298.0780.
(E)-1-((4-Nitrophenyl)sulfonyl)-2-styryl-1H-benzo[d]imidazole (5f).
A yellow oil (18 mg, 22%); 1H NMR (400 MHz, CDCl3) δ 8.27 (d, J = 9.2 Hz, 2H), 8.16–8.03 (m, 3H), 7.97–7.85 (m, 2H), 7.72–7.66 (m, 3H), 7.49–7.37 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 151.3, 151.3, 143.5, 143.1, 141.2, 135.6, 132.9, 130.3, 129.3, 128.4, 128.1, 126.3, 125.9, 125.1, 120.6, 114.0, 113.8; IR (neat) ν 3099, 2923, 2846, 1730, 1625, 1533, 1387, 1346, 1231, 1200, 1167, 1087, 1048, 854, 759, 680 cm−1; HRMS (EI) calcd for C21H15N3O4S, 405.0783; found, 405.0780.
(E)-1-((4-Fluorophenyl)sulfonyl)-2-styryl-1H-benzo[d]imidazole (5g).
A yellow oil (31 mg, 41%); 1H NMR (400 MHz, CDCl3) δ 8.09–8.04 (m, 1H), 7.96–7.88 (m, 4H), 7.71–7.66 (m, 3H), 7.47–7.35 (m, 5H), 7.14–7.08 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 166.3 (d, JC–F = 257.2 Hz), 151.3, 143.0, 140.5, 135.9, 134.4 (d, JC–F = 3.4 Hz), 133.2, 130.0, 129.9, 129.3, 128.0, 125.8, 125.5, 120.3, 117.4, 117.2, 114.2 (d, JC–F = 21.6 Hz); IR (neat) ν 3102, 3066, 3027, 2923, 2855, 1626, 1590, 1514, 1493, 1449, 1382, 1344, 1242, 1200, 1087, 1049, 836, 750, 675 cm−1; HRMS (EI) calcd for C21H15FN2O2S, 378.0838; found, 378.0836.
(E)-N-(4-((2-Styryl-1H-benzo[d]imidazol-1-yl)sulfonyl)phenyl)acetamide (5h).
A yellow solid (24 mg, 29%); m.p. 106.1–107.2 °C; 1H NMR (400 MHz, CDCl3) δ 8.08–8.06 (m, 1H), 7.91 (s, 2H), 7.84 (d, J = 8.8 Hz, 2H), 7.70–7.63 (m, 3H), 7.59 (d, J = 8.8 Hz, 2H), 7.46–7.34 (m, 6H), 2.15 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.7, 151.4, 143.7, 142.9, 140.2, 136.0, 133.3, 132.8, 129.9, 129.2, 128.6, 128.0, 125.6, 125.4, 120.2, 119.6, 114.6, 114.2, 25.0; IR (neat) ν 3316, 3101, 2293, 1697, 1589, 1531, 1376, 1313, 1263, 1230, 1166, 1084, 1046 cm−1; HRMS (EI) calcd for C23H19N3O3S, 417.1147; found, 417.1142.
(E)-6-Methoxy-2-styryl-1-tosyl-1H-benzo[d]imidazole (5i).
A yellow solid (24 mg, 30%); m.p. 132.6–133.6 °C; 1H NMR (400 MHz, CDCl3) δ 7.90–7.80 (m, 2H), 7.77 (d, J = 8.4 Hz, 2H), 7.65–7.61 (m, 3H), 7.56 (d, J = 8.8 Hz, 1H), 7.45–7.36 (m, 3H), 7.23 (d, J = 8.0 Hz, 2H), 7.97 (dd, J1 = 2.4, J2 = 8.8 Hz, 1H), 3.92 (s, 3H), 2.35 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 158.3, 150.4, 146.2, 138.8, 137.2, 136.2, 135.6, 134.3, 130.4, 129.6, 129.2, 127.9, 127.0, 120.5, 114.8, 114.2, 98.5, 56.3, 21.9; IR (neat) ν 3024, 2917, 2846, 1736, 1625, 1596, 1510, 1447, 1378, 1021, 1170, 1089, 1045, 812, 753, 668 cm−1; HRMS (EI) calcd for C23H20N2O3S, 404.1195; found, 404.1198.
(E)-6-Methyl-2-styryl-1-tosyl-1H-benzo[d]imidazole (5j).
A yellow solid (28 mg, 36%); m.p. 183.2–184.7 °C; 1H NMR (400 MHz, CDCl3) δ 7.88 (d, J = 3.0 Hz, 3H), 7.77 (d, J = 8.4 Hz, 2H), 7.66–7.64 (m, 2H), 7.56 (d, J = 8.4 Hz, 1H), 7.46–7.42 (m, 2H), 7.40–7.38 (m, 1H), 7.23 (d, J = 8.0 Hz, 2H), 7.57 (dd, J1 = 1.2, J2 = 8.4 Hz, 1H), 2.52 (s, 3H), 2.34 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 150.8, 146.1, 141.0, 139.5, 136.1, 135.7, 133.6, 130.4, 129.7, 129.2, 128.4, 127.9, 127.0, 127.0, 119.6, 114.8, 114.2, 22.4, 21.9; IR (neat) ν 3390, 3063, 2920, 2846, 1739, 1623, 1593, 1447, 1378, 1200, 1170, 1147, 1089, 1043, 812, 748 cm−1; HRMS (EI) calcd for C23H20N2O2S, 388.1245; found, 388.1245.
(E)-4-Methyl-2-styryl-1-tosyl-1H-benzo[d]imidazole (5k).
A fresh solid (23 mg, 30%); m.p. 165.3–166.3 °C; 1H NMR (400 MHz, CDCl3) δ 7.93 (s, 2H), 7.90 (d, J = 8.0 Hz, 1H), 7.77 (d, J = 8.4 Hz, 2H), 7.69–7.66 (m, 2H), 7.46–7.37 (m, 3H), 7.24–7.20 (m, 3H), 7.15 (d, J = 7.6 Hz, 1H), 2.63 (s, 3H), 2.33 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 150.6, 146.0, 142.2, 139.7, 136.2, 135.6, 133.1, 130.4, 129.7, 129.2, 128.4, 128.0, 127.1, 126.0, 125.2, 115.0, 111.6, 21.9, 16.8; IR (neat) ν 3060, 3027, 2923, 2852, 1636, 1597, 1512, 1447, 1376, 1344, 1200, 1176, 1101, 1016, 972, 814, 759 cm−1; HRMS (EI) calcd for C23H20N2O2S, 388.1245; found, 388.1249.
Acknowledgements
The authors thank the National Natural Science Foundation of China (nos. 21272204) for financial support.
Notes and references
-
(a) I. Bae, H. Han and S. Chang, J. Am. Chem. Soc., 2005, 127, 2038 CrossRef CAS PubMed;
(b) S. H. Cho, E. J. Yoo, I. Bae and S. Chang, J. Am. Chem. Soc., 2005, 127, 16046 CrossRef CAS PubMed.
- For leading reviews on ketenimine chemistry, see:
(a) P. Lu and Y.-G. Wang, Chem. Soc. Rev., 2012, 41, 5687 RSC;
(b) S. H. Kim, S. H. Park, J. H. Choi and S. Chang, Chem. – Asian J., 2011, 6, 2618 CrossRef CAS PubMed;
(c) P. Lu and Y.-G. Wang, Synlett, 2010, 165 CAS;
(d) E. J. Yoo and S. Chang, Curr. Org. Chem., 2009, 13, 1766 CrossRef CAS.
- For selected examples, see:
(a) E. J. Yoo, S. H. Park, S. H. Lee and S. Chang, Org. Lett., 2009, 11, 1155 CrossRef CAS PubMed;
(b) S. H. Cho and S. Chang, Angew. Chem., Int. Ed., 2008, 47, 2836 CrossRef CAS PubMed;
(c) E. J. Yoo, M. Ahlquist, I. Bae, K. B. Sharpless, V. V. Fokin and S. Chang, J. Org. Chem., 2008, 73, 5520 CrossRef CAS PubMed;
(d) S. H. Kim, D. Y. Jung and S. Chang, J. Org. Chem., 2007, 72, 9769 CrossRef CAS PubMed;
(e) E. J. Yoo, M. Ahlquist, S. H. Kim, I. Bae, V. V. Fokin, K. B. Sharpless and S. Chang, Angew. Chem., Int. Ed., 2007, 46, 1730 CrossRef CAS PubMed.
- For selected examples, see:
(a) Y. P. Xing, B. Y. Cheng, J. Wang, P. Lu and Y. G. Wang, Org. Lett., 2014, 16, 4814 CrossRef CAS PubMed;
(b) L. Sun, Y. Zhu, P. Lu and Y. G. Wang, Org. Lett., 2013, 15, 5894 CrossRef CAS PubMed;
(c) J. J. Wang, J. Wang, P. Lu and Y. G. Wang, J. Org. Chem., 2013, 78, 8816 CrossRef CAS PubMed;
(d) Y. Xing, H. Zhao, Q. Shang, J. Wang, P. Lu and Y. G. Wang, Org. Lett., 2013, 15, 2668 CrossRef CAS PubMed;
(e) Z. Jiang, P. Lu and Y. G. Wang, Org. Lett., 2012, 14, 6266 CrossRef CAS PubMed;
(f) J. Wang, J. J. Wang, Y. X. Zhu, P. Lu and Y. G. Wang, Chem. Commun., 2011, 47, 3275 RSC;
(g) W. Z. Song, W. Lu, J. Wang, P. Lu and Y. G. Wang, J. Org. Chem., 2010, 75, 3481 CrossRef CAS PubMed;
(h) S. L. Cui, J. Wang and Y. G. Wang, Org. Lett., 2007, 9, 5023 CrossRef CAS PubMed.
- For selected examples, see:
(a) S. Li, Y. Luo and J. Wu, Org. Lett., 2011, 13, 4312 CrossRef CAS PubMed;
(b) Z. Chen, D. Zheng and J. Wu, Org. Lett., 2011, 13, 848 CrossRef CAS PubMed;
(c) D. P. Chauhan, S. J. Varma, A. Vijeta, P. Banerjee and P. Talukdar, Chem. Commun., 2014, 50, 323 RSC;
(d) W. J. Yao, L. J. Pan, Y. P. Zhang, G. Wang, X. Q. Wang and C. Ma, Angew. Chem., Int. Ed., 2010, 49, 9210 CrossRef CAS PubMed.
- Y. K. Kumar, G. R. Kumar and M. S. Reddy, J. Org. Chem., 2014, 79, 823 CrossRef PubMed.
- D. P. Chauhan, S. J. Varma, A. Vijeta, P. Banerjee and P. Talukdar, Chem. Commun., 2014, 50, 323 RSC.
-
(a) T. R. M. Rauws and B. U. W. Maes, Chem. Soc. Rev., 2012, 41, 2463 RSC;
(b) B. Ojo, P. G. Dunbar, G. J. Durant, P. I. Nagy, J. J. Huzl III, S. Periasamy, D. O. Ngur, A. A. El-Assadi, W. P. Hoss and W. S. Messer Jr., Bioorg. Med. Chem., 1996, 4, 1605 CrossRef CAS;
(c) C. Thominiaux, B. de Bruin, Y. Bramoulle, F. Hinnen, S. Demphel, H. Valette, M. Bottlaender, L. Besret, M. Kassiou and F. Dolle, Appl. Radiat. Isot., 2006, 64, 348 CrossRef CAS PubMed.
- S. K. Alla, R. K. Kumar, P. Sadhu and T. Punniyamurthy, Org. Lett., 2013, 15, 1334 CrossRef CAS PubMed.
-
(a) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed;
(b) J. D. Pata, W. G. Stirtan, S. W. Goldstein and T. A. Steitz, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 10548 CrossRef CAS PubMed;
(c) N. H. Hauel, H. Nar, H. Priebke, U. Ries, J.-M. Stassen and W. Wienen, J. Med. Chem., 2002, 45, 1757 CrossRef CAS PubMed;
(d) D. Agić, M. Hranjec, N. Jajčanin, K. Starčević, G. Karminski-Zamola and M. Abramić, Bioorg. Chem., 2007, 35, 153 CrossRef PubMed;
(e) K. Ishikawa, Y. Kudo, N. Nishida, T. Suemoto, T. Sawada, T. Iwaki and K. Doh-ura, J. Neurochem., 2006, 99, 198 CrossRef CAS PubMed.
- G. Zhang, F.-I. Wu, X. Jiang, P. Sun and C.-H. Cheng, Synth. Met., 2010, 160, 1906 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qo00196j |
|
| This journal is © the Partner Organisations 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)
CH)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) as the eluent to give pure product 4.
3, v/v) as the eluent to give pure product 4.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) as the eluent to give pure product 5.
4, v/v) as the eluent to give pure product 5.




