Direct transformation of amides: a one-pot reductive Ugi-type three-component reaction of secondary amides†
Jian-Feng
Zheng
*,
Xiang-Yang
Qian
and
Pei-Qiang
Huang
*
Department of Chemistry, Fujian Provincial Key Laboratory of Chemical Biology, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005, P. R. China. E-mail: pqhuang@xmu.edu.cn; zjf485@xmu.edu.cn
First published on 22nd May 2015
Abstract
Amides are a class of highly stable carbonyl compounds, which are widely used as reliable synthetic intermediates in both organic synthesis and pharmaceutical chemistry. Thus, the direct transformation of amides into other classes of compounds under mild reaction conditions is highly desirable and in the meantime challenging. An efficient reductive Ugi-type reaction has been established. The method employs common secondary amides as starting materials. The reaction exhibited a broad substrate scope, good chemoselectivity and functional group tolerance and provides rapid access to a variety of functionalized α-acetamidoamides.
Introduction
Amides are widely used as reliable synthetic intermediates in both organic synthesis and pharmaceutical chemistry.1–4 In this context, at a later stage of a synthetic route, mild and chemoselective transformation of an amide intermediate into another class of compound is indispensable. However, due to the high stability of amides, most of the known transformations require harsh conditions that are of low functional group tolerance.3 Thus, indirect methods based on additional synthetic procedures are commonly adopted.4 The prior transformation of amides to thioamides, followed by S-methylation and reductive alkylation,4b,c,d,g or followed by Eschenmoser sulfide contraction4a,h are the two methods that find widespread applications in alkaloid synthesis.With the current interest to develop step-economical5 and sustainable organic synthesis,6 in recent years, the direct, chemoselective, and versatile transformation of amides into other classes of compounds at lower oxidation states has attracted considerable attention.7 Tremendous effort has been devoted to the direct reduction of amides to give amines.8 Significant progress has also been made on the direct transformation of amides into alcohols,9 imines/aldehydes,10 and enamines.11 Moreover, exciting progress has been achieved in recent years on the use of amides as starting materials for the formation of C–C bonds.12–22 The Vilsmeier–Haack12- and Bischler–Napieralski13-type cyclization reactions, direct heteroaromatization,14 reductive functionalization15,16/bis-alkylation17/coupling,18 and deaminative alkylation19 of amides, amide-based rearrangements,20 as well as the reductive nitro-Mannich cyclization of amides21 have been documented. The power of such transformations has been demonstrated by the highly efficient total synthesis of Kopsia alkaloids,22Aspidosperma alkaloids,13b gephyrotoxin,16f and manzamine A.21b However, a method for the direct transformation of common amides to other classes of N-containing compounds is still highly demanding and remains a formidable challenge. Thus, the merging of the concept of direct transformation of amides with other powerful synthetic methodologies to provide other class of N-containing compounds is highly desirable.
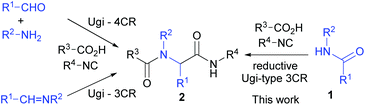
The Ugi four- and three-component reactions23 (Scheme 1) are powerful tools in combinatorial chemistry,24 total synthesis of natural products,23g,h,25 and medicinal chemistry.23g,26 To further improve the synthetic utility and sustainability6 of this powerful reaction, much effort has been devoted to develop new protocols that employ stable starting materials to replace imines and aldehydes. A number of Ugi-type reactions have been reported.27As part of our research program directed toward the development of C–C bond forming reactions starting from common amides,15a,b,e–i,17a,c,18,19b we report herein the first Ugi-type multi-component reaction utilizing common secondary amides as a component.28
Results and discussion
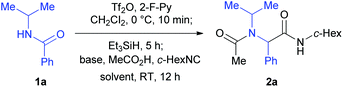
We have previously reported a one-pot reductive cross-coupling of secondary amides with ketones.18 In that method, trifluoromethanesulfonic anhydride (Tf2O)/2-fluoropyridine (2-F-Py) –Et3SiH –NEt3 combination turned out to be effective for generating imine A suitable for the C–C bond forming reaction (Scheme 2). It was envisaged that the in situ generated imine A could also participate in a Ugi-type three-component reaction. It has been reported that the Ugi-type reactions are sensitive to reaction conditions.27f,l The major challenge in attempting an amide-based reductive Ugi-type reaction resided in the compatibility of the reaction conditions for the amide-activation step with those for the one-pot Ugi-type reaction (Scheme 2).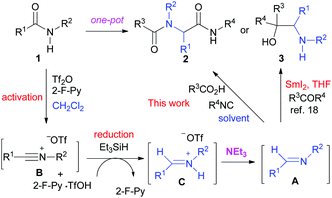 | ||
| Scheme 2 The compatibility of the reaction conditions for the amide-activation step with those of the subsequent steps. | ||
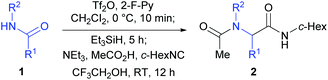
At the outset of our investigation, our previous reaction conditions were adopted.18 Hence, 1a was treated sequentially with trifluoromethanesulfonic anhydride29 (1.3 equiv.) and 2-fluoropyridine30 (1.5 equiv.) in CH2Cl2 at 0 °C for 10 min, and triethylsilane31 (1.1 equiv.) at 0 °C to RT for 5 h. The solvent was then removed and the residue was dissolved in trifluoroethanol (TFE).27l Triethylamine (2.0 equiv.), acetic acid (2.0 equiv.) and cyclohexyl isocyanide (c-hexNC, 1.2 equiv.) were added sequentially and the reaction was then stirred for 12 h. To our delight, under such conditions, the desired product α-acetamidoamide 2a was obtained in 88% yield (Table 1, entry 1). Varying the amount of triethylamine (entries 2–4) or the use of other bases such as the Hünig base or DBU (entries 5 and 6) did not improve the yield.
| Entry | Base (equiv.) | Equiv. of MeCO2H | Solvent | Yielda (%) |
|---|---|---|---|---|
| a Isolated yield. DBU = 1,8-diazabicyclo(5.4.0)undec-7-ene. | ||||
| 1 | Et3N (2.0) | 2.0 | CF3CH2OH | 88 |
| 2 | Et3N (1.0) | 2.0 | CF3CH2OH | 22 |
| 3 | Et3N (3.0) | 2.0 | CF3CH2OH | 82 |
| 4 | Et3N (5.0) | 2.0 | CF3CH2OH | 46 |
| 5 | i-PrNEt2 (2.0) | 2.0 | CF3CH2OH | 73 |
| 6 | DBU (2.0) | 2.0 | CF3CH2OH | 68 |
| 7 | Et3N (2.0) | 1.0 | CF3CH2OH | 63 |
| 8 | Et3N (2.0) | 3.0 | CF3CH2OH | 82 |
| 9 | Et3N (2.0) | 5.0 | CF3CH2OH | 69 |
| 10 | Et3N (2.0) | 2.0 | CH2Cl2 | 57 |
| 11 | Et3N (2.0) | 2.0 | MeOH/CH2Cl2 | 70 |
| 12 | Et3N (2.0) | 2.0 | MeOH | 76 |
Equal amounts of AcOH and Et3N (both 2.0 equiv., entry 1) seemed to be the most efficient combination. Either lowering or increasing the amount of AcOH led to inferior results (entries 7–9), suggesting that maintaining a suitable pH is critical for an efficient reaction. Other solvents such as CH2Cl2 or methanol or a combination of the two were also tested for the Ugi-type reaction but were found to be less efficient than TFE (entries 10–12).
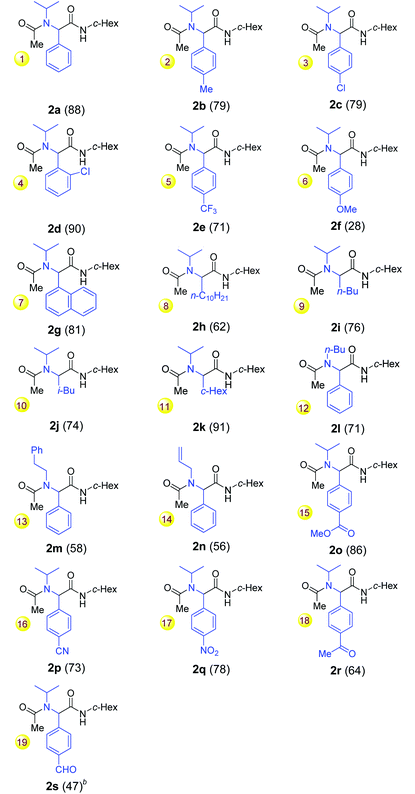
With the optimal conditions for the reductive Ugi-type reaction of secondary amides defined, the scope of the one-pot reaction was explored (Table 2). The reaction tolerated both moderate electron-donating groups (Me) and electron-withdrawing groups at the benzoyl moiety of amides (entries 1–5). However, with p-methoxybenzamide as a substrate, the desired Ugi-adduct 2f was isolated in only 28% (entry 6) along with p-methoxybenzaldehyde. It seemed that the p-methoxybenzimine formed during the reaction was not an effective coupling partner and was probably hydrolyzed during the aqueous workup to give the aldehyde. The use of more equivalents of isocyanide or a prolonged reaction time did not increase the yield. 1-Naphthamide 1g also reacted to give the desired 2g in 81% yield (entry 7). Notably, the reactions of aliphatic amides 1h–k also proceeded smoothly to give the desired products 2h–k in moderate to good yields (entries 8–11). Amides with primary alkyl N-substituents (1l–n) were also suitable substrates and produced the desired α-acetamidoamides albeit in moderate yields (entries 12–14).
We next turned our attention to the chemoselectivity of the reaction. To our delight, the reaction tolerated several sensitive groups, including aromatic ester, cyano, nitro, ketone and aldehyde groups (entries 15–19, 47–86% yields). The latter two groups are usually incompatible with the known Ugi reactions and Ugi-type reactions.
Conclusion
In summary, we have developed an efficient reductive Ugi-type three-component reaction, which features the use of readily available and stable common secondary amides as a component. The reaction tolerated a wide range of substituents on the amides and various functionalized α-acetamidoamides have been synthesized. In view of the widespread use of secondary amides in organic synthesis and medicinal chemistry, their direct transformation into α-acetamidoamides is of great significance.Experimental section
General methods
Melting points were uncorrected and determined on a Büchi M560 Automatic Melting Point apparatus. 1H NMR and 13C NMR spectra were recorded on a Bruker 400 (1H/400 MHz, 13C/100 MHz) or Bruker 500 (1H/500 MHz, 13C/126 MHz) spectrometer, respectively. Chemical shifts (δ) are reported in ppm and respectively referenced to an internal standard of residual chloroform (7.26 ppm for 1H NMR and 77.0 ppm for 13C NMR). Data for 1H NMR are reported as chemical shifts (multiplicity, coupling constant, number of protons). HRFABMS spectra were recorded on a 7.0 T FT-MS. Infrared spectra were recorded with a Nicolet Avatar 330 FT-IR spectrometer using the film or KBr pellet technique. Silica gel (300–400 mesh) was used for flash column chromatography (FC), eluting with an ethyl acetate/hexane mixture. Trifluoromethanesulfonic anhydride (Tf2O) was distilled over phosphorus pentoxide and was stored for no more than a week before re-distillation. Dry dichloromethane was distilled over calcium hydride under an argon atmosphere. All reactions were carried out under an argon atmosphere.General procedure for the one-pot reductive Ugi-type three-component reaction of secondary amides 1
To a cooled (0 °C) solution of secondary amide 1 (0.50 mmol, 1.0 equiv.) and 2-fluoropyridine (65 μL, 0.75 mmol, 1.5 equiv.) in CH2Cl2 (5 mL) were added dropwise Tf2O (110 μL, 0.65 mmol, 1.3 equiv.) and Et3SiH (88 μL, 0.55 mmol, 1.1 equiv.). The reaction mixture was warmed to RT and stirred for 5 h. The solvent was removed through a drying tube charged with anhydrous CaCl2 under reduced pressure. To the concentrate was added 5 mL of CF3CH2OH. To the resulting mixture were added sequentially Et3N (140 μL, 1.00 mmol, 2.0 equiv.), AcOH (60 μL, 1.00 mmol, 2.0 equiv.), and cyclohexyl isocyanide (c-hexNC, 75 μL, 0.60 mmol, 1.2 equiv.). After being stirred for 12 h at RT, the mixture was concentrated under reduced pressure. The residue was taken with ethyl acetate and washed with sat. NaHCO3 and brine. The combined organic phases were dried over anhydrous Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography on silica gel eluting with EtOAc/hexane to afford the desired α-acylamino carboxamide 2.N-Cyclohexyl-2-(N-isopropylacetamido)-2-phenylacetamide (2a)
Following the general procedure, the reduction of amide 1a (82 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/10), the desired product 2a (139 mg, 88%) as a white solid. Mp: 153–155 °C (solvent: MeOH). IR (film) νmax 3293, 3062, 2929, 2852, 1684, 1654, 1626, 1542, 1496, 1449, 1367, 1309, 1201, 1031, 891 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.00–1.13 (m, 3H), 1.09 (d, J = 6.7 Hz, 3H), 1.23–1.40 (m, 2H), 1.37 (d, J = 6.7 Hz, 3H), 1.46–1.61 (m, 3H), 1.78–1.88 (m, 2H), 2.18 (s, 3H), 3.69–3.83 (m, 1H), 4.13 (septet, J = 6.7 Hz, 1H), 4.79 (s, 1H), 6.34 (d, J = 6.4 Hz, 1H), 7.22–7.33 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 21.0, 21.2, 22.7, 24.5, 24.6, 25.4, 32.4, 32.5, 48.2, 50.7, 62.3, 127.6 (2C), 128.5 (3C), 137.0, 169.7, 170.7; HRMS (ESI) calcd for [C19H28N2O2 + Na+]: 339.2048, found: 339.2053.N-Cyclohexyl-2-(N-isopropylacetamido)-2-(p-tolyl)acetamide (2b)
Following the general procedure, the reduction of amide 1b (89 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/10), the desired product 2b (131 mg, 79%) as a white solid. Mp: 166–168 °C (solvent: MeOH). IR (film) νmax 3421, 3296, 3055, 2930, 2854, 1684, 1654, 1624, 1542, 1514, 1448, 1368, 1310, 1273, 1201, 1129 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.00–1.13 (m, 3H), 1.08 (d, J = 6.7 Hz, 3H), 1.24–1.39 (m, 2H), 1.36 (d, J = 6.7 Hz, 3H), 1.47–1.62 (m, 3H), 1.77–1.88 (m, 2H), 2.17 (s, 3H), 2.30 (s, 3H), 3.69–3.81 (m, 1H), 4.12 (septet, J = 6.7 Hz, 1H), 4.75 (s, 1H), 6.28 (d, J = 7.4 Hz, 1H), 7.10 (d, J = 7.9 Hz, 2H), 7.19 (d, J = 7.9 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 21.0 (2C), 21.2, 22.7, 24.5, 24.6, 25.5, 32.5, 32.6, 48.2, 50.7, 62.1, 127.6 (2C), 129.2 (2C), 134.0, 137.2, 169.8, 170.6; HRMS (ESI) calcd for [C20H30N2O2 + Na+]: 353.2205, found: 353.2204.2-(4-Chlorophenyl)-N-cyclohexyl-2-(N-isopropylacetamido)acetamide (2c)
Following the general procedure, the reduction of amide 1c (99 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/10), the desired product 2c (138 mg, 79%) as a white solid. Mp: 172–174 °C (solvent: MeOH). IR (film) νmax 3296, 3060, 2930, 2855, 1654, 1540, 1493, 1442, 1366, 1313, 1194, 1093, 1015, 827 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.99–1.12 (m, 3H), 1.08 (d, J = 6.4 Hz, 3H), 1.21–1.34 (m, 2H), 1.32 (d, J = 6.4 Hz, 3H), 1.44–1.60 (m, 3H), 1.75–1.83 (m, 2H), 2.14 (s, 3H), 3.66–3.77 (m, 1H), 4.10 (septet, J = 6.4 Hz, 1H), 4.73 (s, 1H), 6.45 (d, J = 7.4 Hz, 1H), 7.17–7.25 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 20.9, 21.0, 22.6, 24.4 (2C), 25.3, 32.3, 32.4, 48.2, 50.7, 61.7, 128.5 (2C), 128.7 (2C), 133.2, 135.4, 169.3, 170.8; HRMS (ESI) calcd for [C19H27ClN2O2 + Na+]: 373.1659, found: 373.1658.2-(2-Chlorophenyl)-N-cyclohexyl-2-(N-isopropylacetamido)acetamide (2d)
Following the general procedure, the reduction of amide 1d (99 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/10), the desired product 2d (158 mg, 90%) as a colorless gummy. IR (film) νmax 3286, 3065, 2927, 2854, 1652, 1541, 1439, 1371, 1319, 1201, 1134, 1036, 892, 748 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.89 (d, J = 6.7 Hz, 3H), 0.95–1.12 (m, 3H), 1.26–1.34 (m, 2H), 1.43 (d, J = 6.7 Hz, 3H), 1.48–1.60 (m, 3H), 1.77–1.91 (m, 2H), 2.23 (s, 3H), 3.68–3.83 (m, 1H), 4.10 (septet, J = 6.7 Hz, 1H), 5.11 (d, J = 7.4 Hz, 1H), 5.15 (s, 1H), 7.26–7.32 (m, 2H), 7.37–7.43 (m, 1H), 7.55–7.65 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 20.7, 21.3, 22.5, 24.6, 24.7, 25.5, 32.6 (2C), 48.5, 50.6, 58.6, 127.6, 129.5, 129.6, 130.4, 133.9, 135.2, 167.2, 171.0; HRMS (ESI) calcd for [C19H27ClN2O2 + Na+]: 373.1659, found: 373.1655.N-Cyclohexyl-2-(N-isopropylacetamido)-2-(4-(trifluoromethyl)-phenyl)acetamide (2e)
Following the general procedure, the reduction of amide 1e (116 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/10), the desired product 2e (136 mg, 71%) as a white solid. Mp: 124–126 °C (solvent: MeOH). IR (film) νmax 3298, 3060, 2933, 2855, 1655, 1628, 1542, 1370, 1327, 1165, 1125, 1018 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.03–1.20 (m, 3H), 1.18 (d, J = 6.7 Hz, 3H), 1.25–1.40 (m, 2H), 1.37 (d, J = 6.7 Hz, 3H), 1.48–1.66 (m, 3H), 1.79–1.89 (m, 2H), 2.20 (s, 3H), 3.70–3.86 (m, 1H), 4.18 (septet, J = 6.7 Hz, 1H), 4.86 (s, 1H), 6.79 (d, J = 7.4 Hz, 1H), 7.38 (d, J = 8.2 Hz, 2H), 7.55 (d, J = 8.2 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 21.0, 21.1, 22.8, 24.5, 24.6, 25.4, 32.4, 32.6, 48.4, 51.0, 62.4, 124.0 (q, JC–F = 274.5 Hz), 125.4 (q, JC–F = 3.7 Hz, 2C), 127.3 (2C), 129.6 (q, JC–F = 32.7 Hz), 141.0, 169.4, 171.1; HRMS (ESI) calcd for [C20H27F3N2O2 + Na+]: 407.1922, found: 407.1920.N-Cyclohexyl-2-(N-isopropylacetamido)-2-(4-methoxyphenyl)-acetamide (2f)
Following the general procedure, the reduction of amide 1f (97 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/8), the desired product 2f (48 mg, 28%) as a white solid. Mp: 144–146 °C (solvent: MeOH). IR (film) νmax 3294, 3063, 2924, 2852, 1651, 1618, 1512, 1447, 1369, 1307, 1252, 1181, 1032, 833 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.00–1.14 (m, 3H), 1.07 (d, J = 6.7 Hz, 3H), 1.23–1.39 (m, 2H), 1.37 (d, J = 6.7 Hz, 3H), 1.48–1.64 (m, 3H), 1.78–1.87 (m, 2H), 2.18 (s, 3H), 3.69–3.83 (m, 1H), 3.79 (s, 3H), 4.12 (septet, J = 6.7 Hz, 1H), 4.73 (s, 1H), 6.15 (d, J = 7.4 Hz, 1H), 6.84 (d, J = 8.6 Hz, 2H), 7.25 (d, J = 8.6 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 21.0, 21.4, 22.7, 24.6, 24.7, 25.5, 32.6 (2C), 48.3, 50.6, 55.2, 61.7, 113.9 (2C), 129.2 (3C), 159.0, 169.8, 170.6; HRMS (ESI) calcd for [C20H30N2O3 + Na+]: 369.2154, found: 369.2154.N-Cyclohexyl-2-(N-isopropylacetamido)-2-(naphthalen-1-yl)acetamide (2g)
Following the general procedure, the reduction of amide 1g (107 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/15), the desired product 2g (148 mg, 81%) as a white solid. Mp: 167–168 °C (solvent: MeOH). IR (film) νmax 3422, 3269, 3057, 2931, 2855, 1657, 1626, 1541, 1446, 1370, 1318, 1198, 1132, 1026, 891, 782 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.67 (d, J = 6.7 Hz, 3H), 0.75–1.01 (m, 3H), 1.15–1.26 (m, 2H), 1.37–1.55 (m, 3H), 1.46 (d, J = 6.7 Hz, 3H), 1.58–1.68 (m, 1H), 1.78–1.87 (m, 1H), 2.25 (s, 3H), 3.62–3.81 (m, 1H), 3.97–4.20 (m, 1H), 5.30 (d, J = 5.4 Hz, 1H), 5.81 (s, 1H), 7.40–7.55 (m, 3H), 7.55–7.66 (m, 1H), 7.75–7.92 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 21.1 (2C), 22.9, 24.5 (2C), 25.3, 32.3 (2C), 48.4, 49.8, 58.4, 122.8, 125.4, 125.7, 126.7, 126.9, 128.9, 129.0, 131.2, 131.6, 133.6, 168.6, 170.9; HRMS (ESI) calcd for [C23H30N2O2 + Na+]: 389.2205, found: 389.2204.N-Cyclohexyl-2-(N-isopropylacetamido)dodecanamide (2h)
Following the general procedure, the reduction of amide 1h (107 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/5), the desired product 2h (118 mg, 62%) as a colorless oil. IR (film) νmax 3304, 3060, 2927, 2854, 1652, 1624, 1541, 1450, 1371, 1317, 1195, 1129 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 6.8 Hz, 3H), 1.18–1.36 (m, 27H), 1.53–1.70 (m, 3H), 1.78–1.87 (m, 2H), 1.96–2.03 (m, 1H), 2.08–2.15 (m, 1H), 2.16 (s, 3H), 3.62–3.88 (m, 2H), 3.98 (septet, J = 6.7 Hz, 1H), 7.75 (br s, 1H); 13C NMR (126 MHz, CDCl3) δ 14.1, 20.7, 21.1, 22.6, 23.5, 24.5 (2C), 25.6, 27.0, 29.3, 29.4 (2C), 29.5 (2C), 30.0, 31.9, 32.5, 32.7, 47.6, 50.5, 61.2, 171.7, 172.3; HRMS (ESI) calcd for [C23H44N2O2 + Na+]: 403.3300, found: 403.3298.N-Cyclohexyl-2-(N-isopropylacetamido)hexanamide (2i)
Following the general procedure, the reduction of amide 1i (72 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/5), the desired product 2i (113 mg, 76%) as a colorless oil. IR (film) νmax 3299, 3057, 2932, 2859, 1656, 1538, 1448, 1369, 1317, 1196, 1135 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.83 (t, J = 7.3 Hz, 3H), 1.17 (d, J = 6.7 Hz, 3H), 1.19 (d, J = 6.7 Hz, 3H), 1.09–1.32 (m, 9H), 1.47–1.66 (m, 3H), 1.71–1.85 (m, 2H), 1.89–2.11 (m, 2H), 2.12 (s, 3H), 3.58–3.76 (m, 2H), 3.93 (septet, J = 6.7 Hz, 1H), 7.69 (br s, 1H); 13C NMR (100 MHz, CDCl3) δ 13.9, 20.7, 21.1, 22.5, 22.8, 23.5, 24.5 (2C), 25.6 (2C), 29.2, 29.7, 32.5, 32.7, 47.6, 171.7, 172.2; HRMS (ESI) calcd for [C17H32N2O2 + Na+]: 319.2361, found: 319.2360.N-Cyclohexyl-2-(N-isopropylacetamido)-4-methylpentanamide (2j)
Following the general procedure, the reduction of amide 1j (72 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/5), the desired product 2j (110 mg, 74%) as a white solid. Mp: 102–104 °C (solvent: MeOH). IR (film) νmax 3304, 3057, 2931, 2859, 1654, 1539, 1447, 1370, 1321, 1195, 1127 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.88 (d, J = 6.4 Hz, 3H), 0.91 (d, J = 6.4 Hz, 3H), 1.20 (d, J = 7.0 Hz, 3H), 1.22 (d, J = 7.0 Hz, 3H), 1.09–1.35 (m, 5H), 1.46–1.68 (m, 5H), 1.73–1.84 (m, 2H), 2.06–2.21 (m, 1H), 2.11 (s, 3H), 3.58–3.71 (m, 1H), 3.94 (m, 2H), 7.60 (br s, 1H); 13C NMR (100 MHz, CDCl3) δ 20.7, 21.2, 22.4, 23.0, 23.6, 24.4, 24.5, 25.4, 25.5, 32.5, 32.6, 39.2, 47.5, 50.3, 58.9, 171.7, 171.8; HRMS (ESI) calcd for [C17H32N2O2 + Na+]: 319.2361, found: 319.2358.N,2-Dicyclohexyl-2-(N-isopropylacetamido)acetamide (2k)
Following the general procedure, the reduction of amide 1k (85 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/5), the desired product 2k (147 mg, 91%) as a white solid. Mp: 138–140 °C (solvent: MeOH). IR (film) νmax 3286, 3054, 2929, 2853, 1657, 1540, 1445, 1363, 1329, 1192, 1034 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.61–0.78 (m, 1H), 1.03–1.34 (m, 15H), 1.46–1.82 (m, 10H), 2.12 (s, 3H), 2.54 (m, 1H), 3.21 (m, 1H), 3.56–3.74 (m, 1H), 3.95 (septet, J = 6.7 Hz, 1H), 8.25 (br s, 1H); 13C NMR (100 MHz, CDCl3) δ 20.6, 21.2, 23.5, 24.4 (2C), 25.6, 25.9 (2C), 26.3, 30.4, 30.8, 32.5, 32.6, 35.8, 47.4, 51.1, 67.6, 171.7, 172.0; HRMS (ESI) calcd for [C19H34N2O2 + Na+]: 345.2518, found: 345.2513.2-(N-Butylacetamido)-N-cyclohexyl-2-phenylacetamide (2l)
Following the general procedure, the reduction of amide 1l (89 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/10), the desired product 2l (117 mg, 71%) as a white solid. Mp: 123–125 °C (solvent: MeOH, lit.32 mp: 123 °C). IR (film) νmax 3298, 3064, 2930, 2857, 1634, 1544, 1451, 1417, 1369, 1300, 1194, 1134, 1042, 738, 700 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.73 (t, J = 7.2 Hz, 3H), 0.91–1.46 (m, 9H), 1.51–1.72 (m, 3H), 1.80–1.97 (m, 2H), 2.16 (s, 3H), 3.28 (t, J = 8.0 Hz, 2H), 3.68–3.92 (m, 1H), 5.84 (s, 1H), 5.90 (s, 1H), 7.26–7.46 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 13.4, 20.0, 21.7, 24.7 (2C), 25.5, 31.6, 32.7 (2C), 47.6, 48.5, 62.4, 128.2, 128.6 (2C), 129.3 (2C), 135.7, 168.9, 171.4; HRMS (ESI) calcd for [C20H30N2O2 + Na+]: 353.2199, found: 353.2203.N-Cyclohexyl-2-(N-phenethylacetamido)-2-phenylacetamide (2m)
Following the general procedure, the reduction of amide 1m (113 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/10), the desired product 2m (110 mg, 58%) as a white solid. Mp: 150–152 °C (solvent: MeOH). IR (film) νmax 3297, 3061, 3024, 2929, 2854, 1634, 1541, 1449, 1413, 1221, 1161, 1027 cm−1; 1H NMR (500 MHz, CDCl3) δ 1.07–1.18 (m, 3H), 1.30–1.40 (m, 2H), 1.58–1.70 (m, 3H), 1.89–1.95 (m, 2H), 2.17–2.23 (m, 1H), 2.18 (s, 3H), 2.62–2.72 (m, 1H), 3.43–3.57 (m, 2H), 3.77–3.89 (m, 1H), 5.70 (d, J = 7.4 Hz, 1H), 5.98 (s, 1H), 6.90 (d, J = 7.4 Hz, 2H), 7.14–7.23 (m, 3H), 7.39–7.49 (m, 5H); 13C NMR (126 MHz, CDCl3) δ 21.8, 24.8 (2C), 25.5, 32.8 (2C), 36.2, 48.6, 49.1, 61.8, 126.5, 128.5 (2C), 128.6 (3C), 128.9 (2C), 129.7 (2C), 135.8, 138.3, 168.8, 171.5; HRMS (ESI) calcd for [C24H30N2O2 + Na+]: 401.2205, found: 401.2201.2-(N-Allylacetamido)-N-cyclohexyl-2-phenylacetamide (2n)
Following the general procedure, the reduction of amide 1n (81 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/10), the desired product 2n (88 mg, 56%) as a white solid. Mp: 134–136 °C (solvent: MeOH). IR (film) νmax 3298, 3069, 2929, 2855, 1634, 1544, 1453, 1412, 1316, 1255, 1192, 1040 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.01–1.17 (m, 3H), 1.26–1.38 (m, 2H), 1.52–1.70 (m, 3H), 1.83–1.93 (m, 2H), 2.12 (s, 3H), 3.71–3.85 (m, 1H), 3.89–4.02 (m, 2H), 4.89–4.99 (m, 2H), 5.34–5.49 (m, 1H), 5.86 (d, J = 7.4 Hz, 1H), 6.07 (s, 1H), 7.28–7.38 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 22.0, 24.7 (2C), 25.4, 32.7 (2C), 48.5, 49.4, 61.2, 116.3, 128.3, 128.6 (2C), 129.4 (2C), 134.2, 135.6, 168.6, 172.1; HRMS (ESI) calcd for [C19H26N2O2 + Na+]: 337.1892, found: 337.1888.Methyl-4-(2-(cyclohexylamino)-1-(N-isopropylacetamido)-2-oxoethyl)benzoate (2o)
Following the general procedure, the reduction of amide 1o (111 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/8), the desired product 2o (161 mg, 86%) as a colorless oil. IR (film) νmax 3294, 3057, 2931, 2855, 1723, 1655, 1539, 1439, 1367, 1280, 1189, 1110, 1021, 969, 743 cm−1; 1H NMR (500 MHz, CDCl3) δ 1.03–1.11 (m, 3H), 1.13 (d, J = 6.7 Hz, 3H) 1.25–1.33 (m, 2H), 1.35 (d, J = 6.7 Hz, 3H), 1.46–1.62 (m, 3H), 1.78–1.86 (m, 2H), 2.18 (s, 3H), 3.71–3.81 (m, 1H), 3.87 (s, 3H), 4.15 (septet, J = 6.7 Hz, 1H), 4.84 (s, 1H), 6.62 (d, J = 7.4 Hz, 1H), 7.33 (d, J = 8.4 Hz, 2H), 7.96 (d, J = 8.4 Hz, 2H); 13C NMR (126 MHz, CDCl3) δ 21.0, 21.1, 22.7, 24.4, 24.5, 25.4, 32.4, 32.5, 48.2, 50.9, 52.0, 62.4, 127.1 (2C), 129.3, 129.7 (2C), 142.1, 166.5, 169.3, 171.0; HRMS (ESI) calcd for [C21H30N2O4 + Na+]: 397.2103, found: 397.2106.2-(4-Cyanophenyl)-N-cyclohexyl-2-(N-isopropylacetamido)acetamide (2p)
Following the general procedure, the reduction of amide 1p (94 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/10), the desired product 2p (125 mg, 73%) as a white solid. Mp: 159–160 °C (solvent: MeOH). IR (film) νmax 3295, 3058, 2932, 2854, 2228, 1655, 1540, 1504, 1446, 1370, 1308, 1196, 1170, 1097, 892, 831 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.03–1.23 (m, 3H), 1.20 (d, J = 6.7 Hz, 3H), 1.25–1.37 (m, 2H), 1.34 (d, J = 6.7 Hz, 3H), 1.48–1.64 (m, 3H), 1.77–1.89 (m, 2H), 2.18 (s, 3H), 3.70–3.83 (m, 1H), 4.18 (septet, J = 6.7 Hz, 1H), 4.84 (s, 1H), 6.94 (d, J = 7.4 Hz, 1H); 7.34 (d, J = 8.3 Hz, 2H), 7.57 (d, J = 8.3 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 20.8, 21.1, 22.7, 24.4 (2C), 25.3, 32.3, 32.5, 48.2, 51.1, 62.5, 111.2, 118.4, 127.4 (2C), 132.1 (2C), 142.2, 169.1, 171.1; HRMS (ESI) calcd for [C20H27N3O2 + Na+]: 364.2001, found: 364.1997.N-Cyclohexyl-2-(N-isopropylacetamido)-2-(4-nitrophenyl)acetamide (2q)
Following the general procedure, the reduction of amide 1q (104 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/10), the desired product 2q (141 mg, 78%) as a white solid. Mp: 149–151 °C (solvent: MeOH). IR (film) νmax 3296, 3071, 2931, 2853, 1656, 1605, 1520, 1448, 1347, 1195, 1110, 892, 854 cm−1; 1H NMR (500 MHz, CDCl3) δ 1.04–1.15 (m, 3H), 1.20 (d, J = 6.7 Hz, 3H), 1.24–1.35 (m, 2H), 1.33 (d, J = 6.7 Hz, 3H), 1.46–1.62 (m, 3H), 1.76–1.86 (m, 2H), 2.17 (s, 3H), 3.69–3.81 (m, 1H), 4.18 (septet, J = 6.7 Hz, 1H), 4.86 (s, 1H), 6.99 (d, J = 7.5 Hz, 1H), 7.38 (d, J = 8.8 Hz, 2H), 8.10 (d, J = 8.8 Hz, 2H); 13C NMR (126 MHz, CDCl3) δ 20.7, 21.0, 22.7, 24.3 (2C), 25.3, 32.2, 32.4, 48.2, 51.1, 62.3, 123.4 (2C), 127.4 (2C), 144.2, 146.9, 169.0, 171.2; HRMS (ESI) calcd for [C19H27N3O4 + Na+]: 384.1899, found: 384.1899.2-(4-Acetylphenyl)-N-cyclohexyl-2-(N-isopropylacetamido)acetamide (2r)
Following the general procedure, the reduction of amide 1r (103 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/10), the desired product 2r (115 mg, 64%) as a colorless gummy. IR (film) νmax 3294, 3054, 2930, 2855, 1678, 1538, 1439, 1362, 1310, 1267, 1191 cm−1; 1H NMR (500 MHz, CDCl3) δ 1.02–1.18 (m, 3H), 1.16 (d, J = 6.7 Hz, 3H), 1.25–1.38 (m, 2H), 1.35 (d, J = 6.7 Hz, 3H), 1.46–1.62 (m, 3H), 1.78–1.87 (m, 2H), 2.18 (s, 3H), 2.54 (s, 3H), 3.70–3.83 (m, 1H), 4.17 (septet, J = 6.7 Hz, 1H), 4.84 (s, 1H), 6.74 (d, J = 7.3 Hz, 1H), 7.34 (d, J = 8.2 Hz, 2H), 7.87 (d, J = 8.2 Hz, 2H); 13C NMR (126 MHz, CDCl3) δ 21.0, 21.1, 22.7, 24.4, 24.5, 25.4, 26.4, 32.4, 32.5, 48.2, 51.0, 62.5, 127.1 (2C), 128.4 (2C), 136.2, 142.2, 169.4, 171.0, 197.3; HRMS (ESI) calcd for [C21H30N2O3 + Na+]: 381.2154, found: 381.2152.N-Cyclohexyl-2-(4-formylphenyl)-2-(N-isopropylacetamido)acetamide (2s)
Following the general procedure, the reduction of amide 1s (96 mg, 0.5 mmol) gave, after flash column chromatography on silica gel (eluent: EtOAc/hexane = 1/15), the desired product 2s (81 mg, 47%) as a colorless gummy. IR (film) νmax 3293, 3057, 2930, 2854, 1694, 1660, 1537, 1440, 1370, 1309, 1210 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.06–1.22 (m, 3H), 1.20 (d, J = 6.7 Hz, 3H), 1.27–1.39 (m, 2H), 1.34 (d, J = 6.7 Hz, 3H), 1.48–1.65 (m, 3H), 1.79–1.89 (m, 2H), 2.21 (s, 3H), 3.72–3.86 (m, 1H), 4.19 (septet, J = 6.7 Hz, 1H), 4.88 (s, 1H), 6.84 (d, J = 7.3 Hz, 1H), 7.43 (d, J = 8.2 Hz, 2H), 7.82 (d, J = 8.2 Hz, 2H), 9.97 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 21.0, 21.2, 22.8, 24.5 (2C), 25.4, 32.4, 32.6, 48.3, 51.1, 62.7, 127.5 (2C), 129.8 (2C), 135.5, 143.8, 169.4, 171.1, 191.5; HRMS (ESI) calcd for [C20H28N2O3 + Na+]: 367.1998, found: 367.1996.Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (NSFC, 21332007), Chinese Universities Scientific Fund (No. 20720150048) and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) of the Ministry of Education.Notes and references
- For selected reviews, see: (a) W. N. Speckamp and M. J. Moolenaar, Tetrahedron, 2000, 56, 3817 CrossRef CAS; (b) M. D. Groaning and A. I. Meyers, Tetrahedron, 2000, 56, 9843 CrossRef CAS; (c) N. Toyooka and H. Nemoto, Drugs Future, 2002, 27, 143 CrossRef CAS; (d) N. Toyooka and H. Nemoto, Stud. Nat. Prod. Chem., 2003, 29, 419 CAS; (e) B. E. Maryanoff, H.-C. Zhang, J. H. Cohen, I. J. Turchi and C. A. Maryanoff, Chem. Rev., 2004, 104, 1431 CrossRef CAS PubMed; (f) J. Royer, M. Bonin and L. Micouin, Chem. Rev., 2004, 104, 2311 CrossRef CAS PubMed; (g) P.-Q. Huang, Synlett, 2006, 1133 CrossRef CAS PubMed; (h) A. Yazici and S. G. Pyne, Synthesis, 2009, 339 CAS; (i) A. Yazici and S. G. Pyne, Synthesis, 2009, 513 CAS; (j) M. A. Wijdeven, J. Willemsen and F. P. J. T. Rutjes, Eur. J. Org. Chem., 2010, 2831 CrossRef CAS PubMed; (k) M. Amat, M. Pérez and J. Bosch, Chem. – Eur. J., 2011, 17, 7724 CrossRef CAS PubMed; (l) L.-W. Ye, C. Shu and F. Gagosz, Org. Biomol. Chem., 2014, 12, 1833 RSC. For selected examples, see: (m) W. Zhou, P. Qian, J.-C. Zhao, H. Fang, J.-L. Han and Y. Pan, Org. Lett., 2015, 17, 1160 CrossRef CAS PubMed; (n) B. Peng, X.-L. Huang, L.-G. Xie and N. Maulide, Angew. Chem., Int. Ed., 2014, 53, 8718 CrossRef CAS PubMed; (o) L. Huang, Q. Wang, W. Wu and H. Jiang, J. Org. Chem., 2014, 79, 7734 CrossRef CAS PubMed; (p) B. Peng, D. Geerdink, C. Farès and N. Maulide, Angew. Chem., Int. Ed., 2014, 53, 5462 CrossRef CAS PubMed; (q) A. Jaganathan, R. J. Staples and B. Borhan, J. Am. Chem. Soc., 2013, 135, 14806 CrossRef CAS PubMed; (r) S. Bonazzi, B. Cheng, J. S. Wzorek and D. A. Evans, J. Am. Chem. Soc., 2013, 135, 9338 CrossRef CAS PubMed; (s) Z.-H. Chen, Z.-M. Chen, Y.-Q. Zhang, Y.-Q. Tu and F.-M. Zhang, J. Org. Chem., 2011, 76, 10173 CrossRef CAS PubMed; (t) P. Jakubec, A. F. Kyle, J. Calleja and D. J. Dixon, Tetrahedron Lett., 2011, 52, 6094 CrossRef CAS PubMed; (u) P. M. Lundin and G. C. Fu, J. Am. Chem. Soc., 2010, 132, 11027 CrossRef CAS PubMed; (v) G. A. Molander and L. Jean-Gérard, J. Org. Chem., 2009, 74, 5446 CrossRef CAS PubMed; (w) R. B. Lettan II, C. V. Galliford, C. C. Woodward and K. A. Scheidt, J. Am. Chem. Soc., 2009, 131, 8805 CrossRef PubMed; (x) K. C. Nicolaou, S. M. Dalby and U. Majumder, J. Am. Chem. Soc., 2008, 130, 14942 CrossRef CAS PubMed.
- For a review on the use of amides as directing groups in C–H functionalizations, see: (a) C. Zhu, R. Wang and J. R. Falck, Chem. – Asian J., 2012, 7, 1502 CrossRef CAS PubMed. For selected examples, see: (b) J. He, S.-H. Li, Y.-Q. Deng, H.-Y. Fu, B. N. Laforteza, J. E. Spangler, A. Homs and J.-Q. Yu, Science, 2014, 343, 1216 CrossRef CAS PubMed; (c) A. K. Schoonen, M. Á. Fernández-Ibáñez, M. Fañanás-Mastral, J. F. Teichert and B. L. Feringa, Org. Biomol. Chem., 2014, 12, 36 RSC; (d) L.-S. Zhang, K. Chen, G.-H. Chen, B.-J. Li, S. Luo, Q.-Y. Guo, J.-B. Wei and Z.-J. Shi, Org. Lett., 2013, 15, 10 CrossRef CAS PubMed; (e) K. Smith, G. A. El-Hiti and M. B. Alshammari, J. Org. Chem., 2012, 77, 11210 CrossRef CAS PubMed; (f) J. Ryu, K. Shin, S. H. Park, J. Y. Kim and S. Chang, Angew. Chem., Int. Ed., 2012, 51, 9904 CrossRef CAS PubMed; (g) Q. Chen, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2011, 133, 428 CrossRef CAS PubMed; (h) D. Shabashov, J. R. M. Maldonado and O. Daugulis, J. Org. Chem., 2008, 73, 7818 CrossRef CAS PubMed.
- For a chemoselective reduction of lactams by borane, see: H. C. Brown and P. Heim, J. Org. Chem., 1973, 38, 912 CrossRef CAS.
- (a) K. Shiosaki, In The Eschenmoser Coupling Reaction, in Comprehensive Organic Synthesis, ed. B. M. Trost, I. Fleming and C. H. Heathcock, Oxford, Pergamon, 1991, vol. 2, ch. 3.7 Search PubMed; (b) T. Murai and Y. Mutoh, Chem. Lett., 2012, 41, 2 CrossRef CAS. For selected examples, see: (c) P. Schär and P. Renaud, Org. Lett., 2006, 8, 1569 CrossRef PubMed; (d) A. I. Gerasyuto and R. P. Hsung, J. Org. Chem., 2007, 72, 2476 CrossRef CAS PubMed; (e) B. J. Smith and G. A. Sulikowski, Angew. Chem., Int. Ed., 2010, 49, 1599 CrossRef CAS PubMed; (f) P. Liu, J. H. Seo and S. M. Weinreb, Angew. Chem., Int. Ed., 2010, 49, 2000 CrossRef CAS PubMed; (g) N. R. Perl, N. D. Ide, S. Prajapati, H. H. Perfect, S. G. Durón and D. Y. Gin, J. Am. Chem. Soc., 2010, 132, 1802 CrossRef CAS PubMed; (h) N. D. Koduri, Z. Wang, G. Cannell, K. Cooley, T. M. Lemma, K. Miao, M. Nguyen, B. Frohock, M. Castaneda, H. Scott, D. Albinescu and S. R. Hussaini, J. Org. Chem., 2014, 79, 7405 CrossRef CAS PubMed.
- For a related review involving the concept of step-economy, see: P. A. Wender, V. A. Verma, T. J. Paxton and T. H. Pillow, Acc. Chem. Res., 2008, 41, 40 CrossRef CAS PubMed.
- For leading reviews, see: C.-J. Li and B. M. Trost, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13197 CrossRef CAS PubMed.
- For reviews, see: (a) V. Pace, W. Holzer and B. Olofsson, Adv. Synth. Catal., 2014, 356, 3697 CrossRef CAS PubMed; (b) T. Sato and N. Chida, Org. Biomol. Chem., 2014, 12, 3147 RSC; (c) V. Pace and W. Holzer, Aust. J. Chem., 2013, 66, 507 CAS; (d) D. Seebach, Angew. Chem., Int. Ed., 2011, 50, 96 CrossRef CAS PubMed; (e) C. Madelaine, V. Valerio and N. Maulide, Chem. – Asian J., 2011, 6, 2224 CrossRef CAS PubMed; (f) K. A. DeKorver, H. Li, A. G. Lohse, R. Hayashi, Z.-J. Lu, Y. Zhang and R. P. Hsung, Chem. Rev., 2010, 110, 5064 CrossRef CAS PubMed; (g) A. de Meijere, S. I. Kozhushkov and A. I. Savchenko, J. Organomet. Chem., 2004, 689, 2033 CrossRef CAS PubMed; (h) O. G. Kulinkovich and A. de Meijere, Chem. Rev., 2000, 100, 2789 CrossRef CAS PubMed.
- For recent reviews, see: (a) A. M. Smith and R. Whyman, Chem. Rev., 2014, 114, 5477 CrossRef CAS PubMed; (b) S. Werkmeister, K. Junge and M. Beller, Org. Process Res. Dev., 2014, 18, 289 CrossRef CAS; (c) P. A. Dub and T. Ikariya, ACS Catal., 2012, 2, 1718 CrossRef CAS; (d) D. Addis, S. Das, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 6004 CrossRef CAS PubMed; (e) S. Das, S. Zhou, D. Addis, K. Junge, S. Enthaler and M. Beller, Top. Catal., 2010, 53, 979 CrossRef CAS. For selected examples, see: (f) Y. Motoyama, K. Mitsui, T. Ishida and H. Nagashima, J. Am. Chem. Soc., 2005, 127, 13150 CrossRef CAS PubMed; (g) A. A. Núñez Magro, G. R. Eastham and D. J. Cole-Hamilton, Chem. Commun., 2007, 3154 RSC; (h) S. Hanada, T. Ishida, Y. Motoyama and H. Nagashima, J. Org. Chem., 2007, 72, 7551 CrossRef CAS PubMed; (i) N. Sakai, K. Fujii and T. Konakahara, Tetrahedron Lett., 2008, 49, 6873 CrossRef CAS PubMed; (j) G. Barbe and A. B. Charette, J. Am. Chem. Soc., 2008, 130, 18 CrossRef CAS PubMed; (k) S.-L. Zhou, K. Junge, D. Addis, S. Das and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 9507 CrossRef CAS PubMed; (l) Y. Sunada, H. Kawakami, T. Imaoka, Y. Motoyama and H. Nagashima, Angew. Chem., Int. Ed., 2009, 48, 9511 CrossRef CAS PubMed; (m) S. Hanada, E. Tsutsumi, Y. Motoyama and H. Nagashima, J. Am. Chem. Soc., 2009, 131, 15032 CrossRef CAS PubMed; (n) S. Das, D. Addis, S.-L. Zhou, K. Junge and M. Beller, J. Am. Chem. Soc., 2010, 132, 1770 CrossRef CAS PubMed (correction: J. Am. Chem. Soc., 2010, 132, 4971); (o) S.-H. Xiang, J. Xu, H.-Q. Yuan and P.-Q. Huang, Synlett, 2010, 1829 CAS; (p) J. D. Kirkham, R. J. Butlin and J. P. Harrity, Angew. Chem., Int. Ed., 2012, 51, 6402 CrossRef CAS PubMed; (q) M. Stein and B. Breit, Angew. Chem., Int. Ed., 2013, 52, 2231 CrossRef CAS PubMed; (r) J. A. M. de, L. Torre, K. Grabow, U. Bentrup, K. Junge, S. Zhou, A. Brückner and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 11577 CrossRef PubMed; (s) J. T. Reeves, Z. Tan, M. A. Marsini, Z. S. Han, Y. Xu, D. C. Reeves, H. Lee, B. Z. Lu and C. H. Senanayake, Adv. Synth. Catal., 2013, 355, 47 CrossRef CAS PubMed; (t) T. Zhang, Y. Zhang, W. Zhang and M. Luo, Adv. Synth. Catal., 2013, 355, 2775 CrossRef CAS PubMed; (u) B. Ravinder, S. R. Reddy, A. P. Reddy and R. Bandichhor, Tetrahedron Lett., 2013, 54, 4908 CrossRef CAS PubMed; (v) P.-Q. Huang and H. Geng, Org. Chem. Front., 2015, 2, 150 RSC.
- (a) E. Balaraman, B. Gnanaprakasam, L. J. W. Shimon and D. Milstein, J. Am. Chem. Soc., 2010, 132, 16756 CrossRef CAS PubMed; (b) J. M. John and S. H. Bergens, Angew. Chem., Int. Ed., 2011, 50, 10377 CrossRef CAS PubMed; (c) S. Krackl, C. I. Someya and S. Enthaler, Chem. – Eur. J., 2012, 18, 15267 CrossRef CAS PubMed; (d) M. Szostak, M. Spain, A. J. Eberhart and D. J. Procter, J. Am. Chem. Soc., 2014, 136, 2268 CrossRef CAS PubMed; (e) Y. Kita, T. Higuchi and K. Mashima, Chem. Commun., 2014, 50, 11211 RSC.
- (a) S. Bower, K. A. Kreutzer and S. L. Buchwald, Angew. Chem., Int. Ed., 1996, 35, 1515 CrossRef CAS PubMed; (b) D. J. A. Schedler, J. Li and B. Ganem, J. Org. Chem., 1996, 61, 4115 CrossRef CAS PubMed; (c) J. M. White, A. R. Tunoori and G. I. Georg, J. Am. Chem. Soc., 2000, 122, 11995 CrossRef CAS; (d) J. T. Spletstoser, J. M. White, A. R. Tunoori and G. I. Georg, J. Am. Chem. Soc., 2007, 129, 3408 CrossRef CAS PubMed; (e) Y. M. Choi, M. E. Kim and D. K. An, Bull. Korean Chem. Soc., 2009, 30, 2825 CrossRef CAS; (f) S. Laval, W. Dayoub, A. Favre-Reguillon, P. Demonchaux, G. Mignani and M. Lemaire, Tetrahedron Lett., 2010, 51, 2092 CrossRef CAS PubMed; (g) G. Pelletier, W. S. Bechara and A. B. Charette, J. Am. Chem. Soc., 2010, 132, 12817 CrossRef CAS PubMed; (h) C. Cheng and M. Brookhart, J. Am. Chem. Soc., 2012, 134, 11304 CrossRef CAS PubMed.
- (a) Y. Motoyama, M. Aoki, N. Takaoka, R. Aoto and H. Nagashima, Chem. Commun., 2009, 1574 RSC; (b) A. Volkov, F. Tinnis and H. Adolfsson, Org. Lett., 2014, 16, 680 CrossRef CAS PubMed.
- (a) G. Bélanger, R. Larouche-Gauthier, F. Ménard, M. Nantel and F. Barabé, Org. Lett., 2005, 7, 4431 CrossRef PubMed; (b) G. Bélanger, R. Larouche-Gauthier, F. Ménard, M. Nantel and F. Barabé, J. Org. Chem., 2006, 71, 704 CrossRef PubMed; (c) R. Larouche-Gauthier and G. Bélanger, Org. Lett., 2008, 10, 4501 CrossRef CAS PubMed; (d) F. Lévesque and G. Bélanger, Org. Lett., 2008, 10, 4939 CrossRef PubMed; (e) G. Bélanger, V. Darsigny, M. Doré and F. Lévesque, Org. Lett., 2010, 12, 1396 CrossRef PubMed; (f) G. Bélanger, J. Boudreault and F. Lévesque, Org. Lett., 2011, 13, 6204 CrossRef PubMed; (g) G. Bélanger, G. O'Brien and R. Larouche-Gauthier, Org. Lett., 2011, 13, 4268 CrossRef PubMed; (h) G. Bélanger, M. Dupuis and R. Larouche-Gauthier, J. Org. Chem., 2012, 77, 3215 CrossRef PubMed.
- (a) J. W. Medley and M. Movassaghi, Org. Lett., 2013, 15, 3614 CrossRef CAS PubMed; (b) J. W. Medley and M. Movassaghi, Angew. Chem., Int. Ed., 2012, 51, 4572 CrossRef CAS PubMed.
- (a) M. Movassaghi and M. D. Hill, J. Am. Chem. Soc., 2006, 128, 4592 CrossRef CAS PubMed; (b) M. Movassaghi, M. D. Hill and O. K. Ahmad, J. Am. Chem. Soc., 2007, 129, 10096 CrossRef CAS PubMed; (c) H.-B. Zhou, G.-S. Liu and Z.-J. Yao, J. Org. Chem., 2007, 72, 6270 CrossRef CAS PubMed; (d) Q.-L. Dong, G.-S. Liu, H.-B. Zhou, L. Chen and Z.-J. Yao, Tetrahedron Lett., 2008, 49, 1636 CrossRef CAS PubMed; (e) P. Xu, G.-S. Liu, J. Xi, S.-z. Wang and Z.-J. Yao, Tetrahedron, 2011, 67, 5455 CrossRef CAS PubMed.
- For reductive functionalization of sec-amides: (a) K.-J. Xiao, A.-E. Wang and P.-Q. Huang, Angew. Chem., Int. Ed., 2012, 51, 8314 CrossRef CAS PubMed; (b) P.-Q. Huang, Y.-H. Huang, K.-J. Xiao, Y. Wang and X.-E. Xia, J. Org. Chem., 2015, 80, 2861 CrossRef CAS PubMed. For reductive cyanation of lactams, see: (c) Q. Xia and B. Ganem, Org. Lett., 2001, 3, 485 CrossRef CAS. For reductive functionalization of tert-amides: (d) Y. Oda, T. Sato and N. Chida, Org. Lett., 2012, 14, 950 CrossRef CAS PubMed; (e) K.-J. Xiao, Y. Wang, Y.-H. Huang, X.-G. Wang and P.-Q. Huang, J. Org. Chem., 2013, 78, 8305 CrossRef CAS PubMed; (f) H.-Q. Deng, X.-Y. Qian, Y.-X. Li, J.-F. Zheng, L. Xie and P.-Q. Huang, Org. Chem. Front., 2014, 1, 258 RSC; (g) L.-H. Gao, A.-E Wang and P.-Q. Huang, Sci. China: Chem., 2014, 57, 252 CrossRef; (h) P.-Q. Huang, W. Ou, K.-J. Xiao and A.-E Wang, Chem. Commun., 2014, 50, 8761 RSC; (i) P.-Q. Huang, H. Geng, Y.-S. Tian, Q.-R. Peng and K.-J. Xiao, Sci. China: Chem., 2015, 58, 478 CrossRef CAS; (j) A. Romanens, G. Bélanger, G. O'Brien and R. Larouche-Gauthier, Org. Lett., 2015, 17, 322 CrossRef CAS PubMed.
- For reductive alkylation/functionalization of N-alkoxyamides, see: (a) K. Shirokane, Y. Kurosaki, T. Sato and N. Chida, Angew. Chem., Int. Ed., 2010, 49, 6369 CrossRef CAS PubMed; (b) G. Vincent, R. Guillot and C. Kouklovsky, Angew. Chem., Int. Ed., 2011, 50, 1350 CrossRef CAS PubMed; (c) Y. Yanagita, H. Nakamura, K. Shirokane, Y. Kurosaki, T. Sato and N. Chida, Chem. – Eur. J., 2013, 19, 678 CrossRef CAS PubMed; (d) G. Vincent, D. Karila, G. Khalil, P. Sancibrao, D. Gori and C. Kouklovsky, Chem. – Eur. J., 2013, 19, 9358 CrossRef CAS PubMed; (e) M. Jäkel, J. Qu, T. Schnitzer and G. Helmchen, Chem. – Eur. J., 2013, 19, 16746 CrossRef PubMed; (f) K. Shirokane, T. Wada, M. Yoritate, R. Minamikawa, N. Takayama, T. Sato and N. Chida, Angew. Chem., Int. Ed., 2014, 53, 512 CrossRef CAS PubMed; (g) M. Nakajima, Y. Oda, T. Wada, R. Minamikawa, K. Shirokane, T. Sato and N. Chida, Chem. – Eur. J., 2014, 20, 17565 CrossRef CAS PubMed; (h) M. Nakajima, T. Sato and N. Chida, Org. Lett., 2015, 17, 1696 CrossRef CAS PubMed; (i) K. Shirokane, Y. Tanaka, M. Yoritate, N. Takayama, T. Sato and N. Chida, Bull. Chem. Soc. Jpn., 2015, 88, 522 CrossRef CAS.
- (a) K.-J. Xiao, J.-M. Luo, K.-Y. Ye, Y. Wang and P.-Q. Huang, Angew. Chem., Int. Ed., 2010, 49, 3037 CrossRef CAS PubMed; (b) C. Guérot, B. H. Tchitchanov, H. Knust and E. M. Carreira, Org. Lett., 2011, 13, 780 CrossRef PubMed; (c) H.-H. Huo, X.-E. Xia, H.-K. Zhang and P.-Q. Huang, J. Org. Chem., 2013, 78, 455 CrossRef CAS PubMed.
- P.-Q. Huang, Q.-W. Lang, A.-E. Wang and J.-F. Zheng, Chem. Commun., 2015, 51, 1096 RSC.
- (a) W. S. Bechara, G. Pelletier and A. B. Charette, Nat. Chem., 2012, 4, 228 CrossRef CAS PubMed; (b) K.-J. Xiao, A.-E Wang, Y.-H. Huang and P.-Q. Huang, Asian J. Org. Chem., 2012, 1, 130 CrossRef CAS PubMed; (c) V. Pace, L. Castoldi and W. Holzer, Chem. Commun., 2013, 49, 8383 RSC.
- (a) C. Madelaine, V. Valerio and N. Maulide, Angew. Chem., Int. Ed., 2010, 49, 1583 CrossRef CAS PubMed; (b) V. Valerio, C. Madelaine and N. Maulide, Chem. – Eur. J., 2011, 17, 4742 CrossRef CAS PubMed; (c) B. Peng, D. H. O'Donovan, I. D. Jurberg and N. Maulide, Chem. – Eur. J., 2012, 18, 16292 CrossRef CAS PubMed; (d) B. Peng, D. Geerdink and N. Maulide, J. Am. Chem. Soc., 2013, 135, 14968 CrossRef CAS PubMed; (e) V. Valerio, D. Petkova, C. Madelaine and N. Maulide, Chem. – Eur. J., 2013, 19, 2606 CrossRef CAS PubMed.
- (a) P. Jakubec, A. Hawkins, W. Felzmann and D. J. Dixon, J. Am. Chem. Soc., 2012, 134, 17482 CrossRef CAS PubMed; (b) A. W. Gregory, A. Chambers, A. Hawkins, P. Jakubec and D. J. Dixon, Chem. – Eur. J., 2015, 21, 111 CrossRef CAS PubMed.
- (a) P. Magnus, L. Gazzard, L. Hobson, A. H. Payne, T. J. Rainey, N. Westlund and V. Lynch, Tetrahedron, 2002, 58, 3423 CrossRef CAS; (b) P. Magnus, L. A. Hobson, N. Westlund and V. Lynch, Tetrahedron Lett., 2001, 42, 993 CrossRef CAS.
- (a) I. Ugi, R. Meyr, U. Fetzer and C. Steinbrückner, Angew. Chem., 1959, 71, 386 Search PubMed; (b) I. Ugi and C. Steinbrückner, Angew. Chem., 1960, 72, 267 CrossRef CAS PubMed; (c) I. Ugi, Angew. Chem., Int. Ed. Engl., 1962, 74, 9 CrossRef CAS PubMed; (d) M. M. Bowers, P. Carroll and M. M. Joullié, J. Chem. Soc., Perkin Trans. 1, 1989, 857 RSC. For reviews on the Ugi reactions and Ugi-type reactions, see: (e) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef; (f) I. Ugi, B. Werner and A. Dömling, Molecules, 2003, 8, 53 CrossRef PubMed; (g) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (h) L. El Kaim and L. Grimaud, Tetrahedron, 2009, 65, 2153 CrossRef CAS PubMed; (i) S. S. van Berkel, B. G. M. Bögels, M. A. Wijdeven, B. Westermann and F. P. J. T. Rutjes, Eur. J. Org. Chem., 2012, 3543 CrossRef CAS PubMed; (j) G. C. Tron, Eur. J. Org. Chem., 2013, 1849 CrossRef CAS PubMed; (k) S. Brauch, S. S. Van Berkel and B. Westermann, Chem. Soc. Rev., 2013, 42, 4948 RSC.
- E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234 CrossRef CAS PubMed.
- (a) W. R. Gutekunst and P. S. Baran, J. Org. Chem., 2014, 79, 2430 CrossRef CAS PubMed; (b) O. Pando, S. Stark, A. Denkert, A. Porzel, R. Preusentanz and L. A. Wessjohann, J. Am. Chem. Soc., 2011, 133, 7692 CrossRef CAS PubMed; (c) S. Takiguchi, T. Iizuka, Y. Kumakura, K. Murasaki, N. Ban, K. Higuchi and T. Kawasaki, J. Org. Chem., 2010, 75, 1126 CrossRef CAS PubMed; (d) J. Isaacson and Y. Kobayashi, Angew. Chem., Int. Ed., 2009, 48, 1845 CrossRef CAS PubMed; (e) X.-C. Li and S. J. Danishefsky, J. Am. Chem. Soc., 2008, 130, 5446 CrossRef CAS PubMed; (f) A. Endo, A. Yanagisawa, M. Ane, S. Tohma, T. Kan and T. Fukuyama, J. Am. Chem. Soc., 2002, 124, 6552 CrossRef CAS PubMed.
- I. Akritopoulou-Zanze, Curr. Opin. Chem. Biol., 2008, 12, 324 CrossRef CAS PubMed.
- (a) L. Moni, L. Banfi, A. Basso, L. Carcone, M. Rasparini and R. Riva, J. Org. Chem., 2015, 80, 3411 CrossRef CAS PubMed; (b) F. Drouet, G. Masson and J.-P. Zhu, Org. Lett., 2013, 15, 2854 CrossRef CAS PubMed; (c) M. Rueping and C. Vila, Org. Lett., 2013, 15, 2092 CrossRef CAS PubMed; (d) C. Vila and M. Rueping, Green Chem., 2013, 15, 2056 RSC; (e) L. Xia, S. Li, R. Chen, K. Liu and X. Chen, J. Org. Chem., 2013, 78, 3120 CrossRef CAS PubMed; (f) B. Beck, S. Srivastava, K. Khoury, E. Herdtweck and A. Dömling, Mol. Diversity, 2010, 14, 479 CrossRef CAS PubMed; (g) A. Znabet, M. M. Polak, E. Janssen, F. J. J. de Kanter, N. J. Turner, R. V. A. Orru and E. Ruijter, Chem. Commun., 2010, 46, 7918 RSC; (h) T. Ngouansavanh and J.-P. Zhu, Angew. Chem., Int. Ed., 2007, 46, 5775 CrossRef CAS PubMed; (i) V. G. Nenajdenko, A. V. Gulevich and E. S. Balenkova, Tetrahedron, 2006, 62, 5922 CrossRef CAS PubMed; (j) P. Janvier, H. Bienaym and J.-P. Zhu, Angew. Chem., Int. Ed., 2002, 41, 4291 CrossRef CAS; (k) A. Fayol and J.-P. Zhu, Angew. Chem., Int. Ed., 2002, 41, 3633 CrossRef CAS; (l) Y. B. Kim, E. H. Choi, G. Keum, S. B. Kang, D. H. Lee, H. Y. Koh and Y. Kim, Org. Lett., 2001, 3, 4149 CrossRef CAS PubMed.
- During the submission of this manuscript, a one-pot sequential lactam reduction/Joullié–Ugi reaction appeared: P. Szcześniak, E. Maziarz, S. Stecko and B. Furman, J. Org. Chem., 2015, 80, 3621 CrossRef PubMed.
- For a recent review on Tf2O, see: I. L. Baraznenok, V. G. Nenajdenko and E. S. Balenkova, Tetrahedron, 2000, 56, 3077 CrossRef CAS.
- J. W. Medley and M. Movassaghi, J. Org. Chem., 2009, 74, 1341 CrossRef CAS PubMed.
- (a) J. L. Fry, J. Chem. Soc., Chem. Commun., 1974, 45 RSC; (b) J. L. Fry and R. A. Ott, J. Org. Chem., 1981, 46, 602 CrossRef CAS; (c) ref. 10g; ; (d) D. N. Kursanov, Z. N. Parnes and N. M. Loim, Synthesis, 1974, 633 CrossRef CAS; (e) ref. 1g .
- M. Ghavami, M. Koohi and M. Z. Kassaee, J. Chem. Sci., 2013, 125, 1347 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR and 13C NMR spectra of the products 2a–2s. See DOI: 10.1039/c5qo00146c |
| This journal is © the Partner Organisations 2015 |