I2-catalyzed one-pot synthesis of pyrrolo[1,2-a]quinoxaline and imidazo[1,5-a]quinoxaline derivatives via sp3 and sp2 C–H cross-dehydrogenative coupling†
Zeyuan
Zhang
a,
Caixia
Xie
a,
Xiaochen
Tan
a,
Gaolei
Song
a,
Leilin
Wen
a,
He
Gao
a and
Chen
Ma
*ab
aSchool of Chemistry and Chemical Engineering, Shandong University, Jinan, 250100, P R China
bState Key Laboratory of Natural and Biomimetic Drugs, Peking University, Beijing, 100191, P R China. E-mail: chenma@sdu.edu.cn
First published on 3rd June 2015
Abstract
An efficient I2-catalyzed cascade coupling protocol was developed for the synthesis of pyrrolo[1,2-a]quinoxaline and imidazo[1,5-a]quinoxaline derivatives via sp3 and sp2 C–H cross-dehydrogenative coupling. A nontoxic, readily available catalyst, I2, and an oxidant, DMSO, were used in this metal-free process. The target compounds were obtained in good-to-excellent yields with a broad substrate scope.
Cross-dehydrogenative coupling (CDC) has recently emerged as an ideal method for the formation of carbon–carbon (C–C)1 and carbon–heteroatom (C–X)2 bonds. Because of its substantial benefits for organic synthesis, this approach has garnered significant attention from the chemical community. However, most of the studied CDC methods suffer from expensive and detrimental metal precursors, harsh reaction conditions, critical product isolation procedures, and unsatisfactory product yields, thus limiting their use in green chemistry. Therefore, it is highly desirable to develop a convenient and efficient approach for CDC.
DMSO has received significant attention since it was first reported in 1957 as an oxidant.3 DMSO is an inexpensive, nontoxic, and readily available oxidant for various organic transformations under mild and convenient reaction conditions.4
Although acid catalysis remains the most widely used type of catalysis, it suffers from serious health and safety problems. Therefore, the development of a safe and atom-efficient acid-catalyzed organic process is one of the most important challenges in green chemistry. I2 is a mild, inexpensive, nontoxic, and readily available Lewis acid with enhanced utility in several organic transformations.5 Moreover, most I2-catalyzed reactions involve multicomponent or domino reaction sequences,6 and forming several bonds in a single operation is one of the important synthetic goals for molecular diversity and complexity.
Recently, pyrrolo[1,2-a]quinoxaline and imidazo[1,5-a]quinoxaline derivatives have received considerable attention for their biological activities such as 5-HTR affinities,7 anticancer,8in vitro antiparasitic,9 and potential nonpeptide glucagon receptor antagonist activities.10
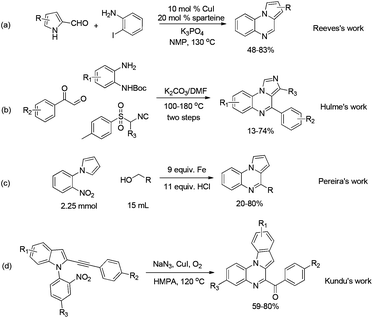
Consequently, many synthetic protocols have been developed to synthesize pyrrolo[1,2-a]quinoxaline or imidazo[1,5-a]quinoxaline derivatives. Reeves et al. reported the synthesis of these tricyclic compounds in N-methylpyrrolidone (NMP) from 1H-pyrrole-2-carbaldehyde and 2-iodoaniline in the presence of CuI and sparteine (Scheme 1a).11a Hulme and co-worker reported a multicomponent reaction for the synthesis of imidazo[1,5-a]quinoxaline derivatives from isonitrile, 2-oxo-2-phenylacetaldehyde, and tert-butyl(2-aminophenyl)carbamate via multiple steps (Scheme 1b).11b Pereira and Thiéry developed a one-pot reaction for synthesizing pyrrolo[1,2-a]quinoxalines from 1-(2-nitrophenyl)pyrroles and various alcohols using excess of iron powder and HCl (Scheme 1c).11c Kundu et al. reported the diversity-oriented synthesis of indoloquinoxalines from 1-(2-nitroaryl)-2-alkynylindoles and NaN3 in hexamethylphosphoramide (HMPA) using CuI as the catalyst (Scheme 1d).11d However, these methods suffer from some disadvantages such as complex manipulation11b and the use of hazardous substrates11b,d or transition-metal catalysts.11a,c,d
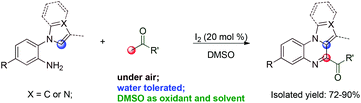
We have been developing the direct synthesis of heterocyclic systems using tandem reactions.12 Herein, we report a cascade coupling protocol for the synthesis of pyrrolo[1,2-a]quinoxaline and imidazo[1,5-a]quinoxaline derivatives using I2 as the catalyst and DMSO as the oxidant via sp3 and sp2 C–H CDC (Scheme 2).
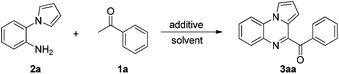
The reaction conditions were optimized using acetophenone 1a and tryptamine 2a as the model substrates, and the results are summarized in Table 1. First, several types of solvents were investigated under a N2 atmosphere; no product was detected until DMSO was used (Table 1, entries 1–6). Then, several types of iodide reagents such as N-iodosuccinimide (NIS), KI, and tetrabutylammonium iodide (TBAI) were screened; no desired product was obtained (Table 1, entries 7–9). The dosages of I2 were also investigated, 20 mol% I2 resulted in 87% yield, while 15 mol% I2 significantly lowered the yield (Table 1, entries 6 and 10–15). Furthermore, a small amount of water and air did not significantly affect the reaction (Table 1, entries 16 and 17). Low yield (3aa) was obtained when 1a and 2a were added simultaneously (Table 1, entry 18).
| Entry | Additive (mol%) | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), additive, and solvent (2 mL), in a sealed tube at 120 °C under a N2 atmosphere for 6 h, and then adding 2a (0.5 mmol). b Yield of the isolated product. c 1% water content. d Under air. e Reaction conditions: 1a (0.5 mmol), 2a (0.5 mmol), I2 (20 mol%), and solvent (2 mL), in a sealed tube at 120 °C under an air atmosphere for 6 h. f DMF = N,N-dimethylformamide, DMA = N,N-dimethylacetamide, OX = o-xylene, NMP = N-methylpyrrolidone, DMSO = dimethyl sulfoxide. | |||
| 1 | I2 (100) | DMF | N.D. |
| 2 | I2 (100) | DMA | N.D. |
| 3 | I2 (100) | OX | N.D. |
| 4 | I2 (100) | PhCl | N.D. |
| 5 | I2 (100) | NMP | N.D. |
| 6 | I2 (100) | DMSO | 85 |
| 7 | NIS (20) | DMSO | Trace |
| 8 | KI (20) | DMSO | N.D. |
| 9 | TBAI (20) | DMSO | N.D. |
| 10 | I2 (120) | DMSO | 83 |
| 11 | I2 (80) | DMSO | 85 |
| 12 | I2 (60) | DMSO | 87 |
| 13 | I2 (40) | DMSO | 87 |
| 14 | I 2 (20) | DMSO | 87 |
| 15 | I2 (15) | DMSO | 80 |
| 16 | I2 (20) | DMSOc | 86 |
| 17 | I 2 (20) | DMSO | 87 |
| 18e | I2 (20) | DMSO | 75 |
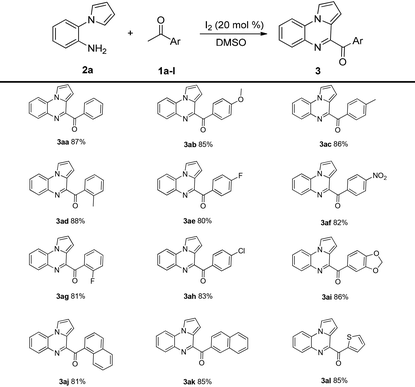
With the optimized reaction conditions, the substrate scope of this method was investigated. As shown in Table 2, diverse aromatic ketones 1a–l were investigated in the reaction with 2-(1H-pyrrol-1-yl)aniline 2a; the corresponding products were obtained in good-to-excellent yields. The yields increased in the presence of electron-donating substituents on the aryl ring. Low yields were obtained when electron-withdrawing substituents were present on the aryl ring. Notably, heterocycles such as 1-(thiophen-2-yl)ethanone and 1-(benzodioxol-5-yl)ethanone also participated in the reaction, affording the corresponding products 3al and 3ai in 85% and 86% yields, respectively. Moreover, sterically hindered 2-acetylnaphthalene and 1-acetylnaphthalene also furnished the desired products 3aj and 3ak in 81% and 85% yields, respectively.
| a Reaction conditions: 1 (0.5 mmol), I2 (20 mol%), and solvent (2 mL), in a sealed tube at 120 °C under a N2 atmosphere for 6 h, and then adding 2 (0.5 mmol). |
|---|
|
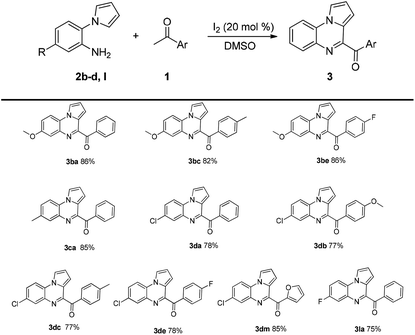
Next, the substrate scope of 2-(1H-pyrrol-1-yl)aniline 2b–d was investigated, and the results are shown in Table 3. The target compounds were obtained in good yields. The yields decreased in the presence of electron-withdrawing substituents on the benzene ring attached to 2-(1H-pyrrol-1-yl)aniline 2, while the electron-donating substituents did not affect the yields of the corresponding products.
| a Reaction conditions: 1 (0.5 mmol), I2 (20 mol%), and solvent (2 mL), in a sealed tube at 120 °C under a N2 atmosphere for 6 h, and then adding 2 (0.5 mmol). |
|---|
|
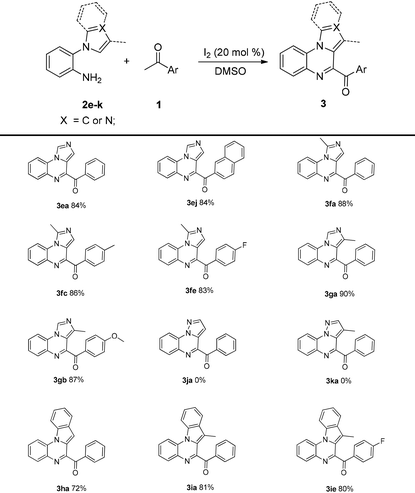
Then, the substrate scope of 2-(1H-imidazol-1-yl)aniline and 2-(1H-indol-1-yl)aniline 2e–i were investigated; the corresponding products were obtained in good-to-excellent yields. Interestingly, considerable yields (3ea and 3ej) were obtained when 2-(1H-imidazol-1-yl)aniline 2e was used. This result could not be achieved in a previous study.13 Disappointingly, 3ja and 3ka could not be obtained by this method (Table 4).
| a Reaction conditions: 1 (0.5 mmol), I2 (20 mol%), and solvent (2 mL), in a sealed tube at 120 °C under a N2 atmosphere for 6 h, and then adding 2 (0.5 mmol). |
|---|
|
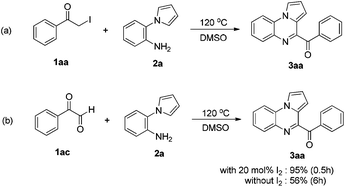
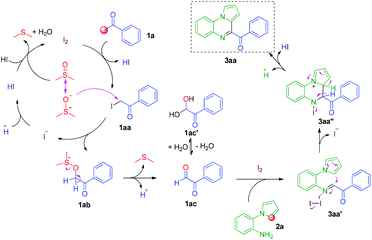
To elucidate the mechanism of the reaction, several control experiments were conducted (Scheme 3). In the absence of I2, possible intermediate 1aa reacted with 2a, affording 3aa in good yield. The target molecule 3aa was also obtained from possible intermediate 1ac by reacting with 2a in the presence of I2.
Based on the above control experiments, a possible mechanism for this reaction is shown in Scheme 4. First, the reaction of acetophenone 1a and I2 affords α-iodoketone 1aa and HI. Then, the oxidation of α-iodoketone 1aa with DMSO affords phenylglyoxal 1ac,14 thus releasing by-products HI and dimethyl sulfide. Then, the I2-catalyzed cyclization of phenylglyoxal 1ac with 2-(1H-pyrrol-1-yl)aniline 2a affords 4-benzoylpyrrolo[1,2-a]quinoxaline 3aa. Finally, the oxidation of the by-product HI with DMSO affords I2, thus releasing H2O and dimethyl sulfide and completing the catalytic cycle.
An eco-friendly method was developed for the synthesis of pyrrolo[1,2-a]quinoxaline and imidazo[1,5-a]quinoxaline derivatives via sp3 and sp2 C–H CDC. A nontoxic, readily available catalyst, I2, and an oxidant, DMSO, were used. This metal-free method has a broad substrate scope, and the target compounds were obtained in good-to-excellent yields. Studies on the application of this method to the synthesis of pharmaceutical compounds are in progress.
Acknowledgements
We are grateful to the National Science Foundation of China (No. 21172131) and the State Key Laboratory of Natural and Biomimetic Drugs of Peking University (No. K20140208) for financial support to this research.Notes and references
- (a) C. J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed; (b) Z. Li and C. J. Li, J. Am. Chem. Soc., 2006, 128, 56 CrossRef CAS PubMed; (c) Z. Li and C. J. Li, J. Am. Chem. Soc., 2005, 127, 6968 CrossRef CAS PubMed; (d) Z. Li and C. J. Li, J. Am. Chem. Soc., 2005, 127, 3672 CrossRef CAS PubMed; (e) Z. Li and C. J. Li, Org. Lett., 2004, 6, 4997 CrossRef CAS PubMed; (f) Z. Li and C. J. Li, J. Am. Chem. Soc., 2004, 126, 11810 CrossRef CAS PubMed; (g) Y. Z. Li, B. J. Li, X. Y. Lu, S. Lin and Z. J. Shi, Angew. Chem., Int. Ed., 2009, 48, 3817 CrossRef CAS PubMed; (h) B. J. Li, S. L. Tian, Z. Fang and Z. J. Shi, Angew. Chem., Int. Ed., 2008, 47, 1115 CrossRef CAS PubMed; (i) W. Shi, C. Liu and A. W. Lei, Chem. Soc. Rev., 2011, 40, 2761 RSC; (j) C. He, S. Guo, J. Ke, J. Hao, H. Xu, H. Y. Chen and A. W. Lei, J. Am. Chem. Soc., 2012, 134, 5766 CrossRef CAS PubMed; (k) M. Gao, C. He, H. Y. Chen, R. P. Bai, B. Cheng and A. W. Lei, Angew. Chem., Int. Ed., 2013, 125, 7096 CrossRef PubMed.
- (a) C. G. Espino, K. W. Fiori, M. Kim and J. DuBois, J. Am. Chem. Soc., 2004, 126, 15378 CrossRef CAS PubMed; (b) K. W. Fiori and J. DuBois, J. Am. Chem. Soc., 2007, 129, 562 CrossRef CAS PubMed; (c) M. Kim, J. V. Mulcahy, C. G. Espino and J. DuBois, Org. Lett., 2006, 8, 1073 CrossRef CAS PubMed; (d) C. G. Liang, F. Robert-Peillard, C. Fruit, P. Müller, R. H. Dodd and P. Dauban, Angew. Chem., Int. Ed., 2006, 45, 4641 CrossRef CAS PubMed; (e) H. Lebel and K. Huard, Org. Lett., 2007, 9, 639 CrossRef CAS PubMed; (f) R. P. Reddy and H. M. L. Davies, Org. Lett., 2006, 8, 5013 CrossRef CAS PubMed; (g) Y. P. Zhu, M. C. Liu, Q. Cai, F. C. Jia and A. X. Wu, Chem. – Eur. J., 2013, 19, 10132 CrossRef CAS PubMed.
- N. Kornblum, J. W. Powers, G. J. Anderson, W. J. Jones, H. O. Larson, O. Levand and W. M. Weaver, J. Am. Chem. Soc., 1957, 79, 6562 CrossRef CAS.
- (a) F. Jia, Y. Zhu, M. Liu, M. Lian, Q. Gao, Q. Cai and A. Wu, Tetrahedron, 2013, 69, 7038 CrossRef CAS PubMed; (b) Z. Fei, Y. Zhu, M. Liu, F. Jia and A. Wu, Tetrahedron Lett., 2013, 54, 1222 CrossRef CAS PubMed; (c) Y. Zhu, Z. Fei, M. Liu, F. Jia and A. Wu, Org. Lett., 2013, 15, 378 CrossRef CAS PubMed; (d) Y. Zhu, F. Jia, M. Liu and A. Wu, Org. Lett., 2013, 14, 4414 CrossRef PubMed; (e) Y. Zhu, M. Liu, F. Jia, J. Yuan, Q. Gao, M. Lian and A. Wu, Org. Lett., 2013, 14, 3392 CrossRef PubMed; (f) W. Ge and Y. Wei, RSC Adv., 2013, 3, 10817 RSC; (g) W. Ge, X. Zhu and Y. Wei, Green Chem., 2012, 14, 2066 RSC; (h) Y. Ashikari, A. Shimizu, T. Nokami and J. Yoshida, J. Am. Chem. Soc., 2013, 135, 16070 CrossRef CAS PubMed.
- (a) Y. F. Liao, P. C. Jiang, S. P. Chen, H. G. Qi and G. J. Deng, Green Chem., 2013, 15, 3302 RSC; (b) P. Katrun, C. Mueangkaew, M. Pohmakotr, V. Reutrakul, T. Jaipetch, D. Soorukram and C. Kuhakarn, J. Org. Chem., 2014, 79, 1778 CrossRef CAS PubMed; (c) A. K. Verma, V. Rustagi, T. Aggarwal and A. P. Singh, J. Org. Chem., 2010, 75, 7691 CrossRef CAS PubMed; (d) H. A. Du, X. G. Zhang, R. Y. Tang and J. H. Li, J. Org. Chem., 2009, 74, 7844 CrossRef CAS PubMed; (e) Z. He, H. Li and Z. Li, J. Org. Chem., 2010, 75, 4636 CrossRef CAS PubMed; (f) C. Masdeu, E. Gomez, N. A. O. Williams and R. Lavilla, Angew. Chem., Int. Ed., 2007, 46, 3043 CrossRef CAS PubMed; (g) B. Quiclet-Sire and S. Z. Zard, Chem. Commun., 2006, 1831 RSC; (h) N. K. Downer-Riley and Y. A. Jackson, Tetrahedron, 2007, 63, 10276 CrossRef CAS PubMed; (i) F. Shibahara, A. Kitagawa, E. Yamaguchi and T. Murai, Org. Lett., 2006, 8, 5621 CrossRef CAS PubMed.
- (a) L. Y. Zeng and C. Cai, J. Comb. Chem., 2010, 12, 35 CrossRef CAS PubMed; (b) P. Zalavadiya, S. Tala, J. Akbari and H. Joshi, Arch. Pharm., 2009, 342, 469 CrossRef CAS PubMed; (c) H. S. Wang and J. E. Zeng, Chin. J. Chem., 2008, 26, 175 CrossRef CAS PubMed; (d) M. Kidwai and P. Mothsra, Tetrahedron Lett., 2006, 47, 5029 CrossRef CAS PubMed; (e) L. Y. Zeng and C. Cai, Org. Biomol. Chem., 2010, 8, 4803 RSC; (f) X. S. Wang, Q. Li, J. R. Wu and S. J. Tu, J. Comb. Chem., 2009, 11, 433 CrossRef CAS PubMed; (g) X. S. Wang, Q. Li, J. Zhou and S. J. Tu, J. Heterocycl. Chem., 2009, 46, 1222 CrossRef CAS PubMed; (h) X. S. Wang, J. Zhou, M. Y. Yin, K. Yang and S. J. Tu, J. Comb. Chem., 2010, 12, 266 CrossRef CAS PubMed; (i) A. T. Khan, M. M. Khan and K. K. R. Bannuru, Tetrahedron, 2010, 66, 7762 CrossRef CAS PubMed.
- (a) H. Prunier, S. Rault, J. C. Lancelot, M. Robba, P. Renard, P. Delagrange, B. Pfeiffer, D. H. Caignard, R. Misslin and M. Hamon, J. Med. Chem., 1997, 40, 1808 CrossRef CAS PubMed; (b) S. Butini, R. Budriesi, M. Hamon, E. Morelli, S. Gemma, M. Brindisi, G. Borrelli, E. Novellino, I. Fiorini, P. Ioan, A. Chiarini, A. Cagnotto, T. Mennini, C. Fracasso, S. Caccia and G. Campiani, J. Med. Chem., 2009, 52, 6946 CrossRef CAS PubMed.
- G. Moarbess, C. Deleuze-Masquefa, V. Bonnard, S. Gayraud-Paniagua, J. R. Vidal, F. Bressolle, F. Pinguet and P. A. Bonnet, Bioorg. Med. Chem., 2008, 16, 6601 CrossRef CAS PubMed.
- J. Guillon, E. Mouray, S. Moreau, C. Mullie, I. Forfar, V. Desplat, S. Belisle-Fabre, N. Pinaud, F. Ravanello, A. Le-Naour, J. M. Leger, G. Gosmann, C. Jarry, G. Deleris, P. Sonnet and P. Grellier, Eur. J. Med. Chem., 2011, 46, 2310 CrossRef CAS PubMed.
- J. Guillon, P. Dallemagne, B. Pfeiffer, P. Renard, D. Manechez, A. Kervran and S. Rault, Eur. J. Med. Chem., 1998, 33, 293 CrossRef CAS.
- (a) J. T. Reeves, D. R. Fandrick, Z. Tan, J. J. Song, H. Lee, N. K. Yee and C. H. Senanayake, J. Org. Chem., 2010, 75, 992 CrossRef CAS PubMed; (b) F. D. Moliner and C. Hulme, Org. Lett., 2012, 14, 1354 CrossRef PubMed; (c) M. F. Pereira and V. Thiéry, Org. Lett., 2012, 14, 4754 CrossRef CAS PubMed; (d) S. Samala, R. K. Arigela, R. Kant and B. Kundu, J. Org. Chem., 2014, 79, 2491 CrossRef CAS PubMed.
- (a) A. P. Huang, Y. M. Chen, Y. G. Zhou, W. Guo, X. D. Wu and M. Chen, Org. Lett., 2013, 15, 5480 CrossRef CAS PubMed; (b) Y. M. Zhao, Y. M. Wu, J. Jia, D. J. Zhang and C. Ma, J. Org. Chem., 2012, 77, 8501 CrossRef CAS PubMed; (c) B. C. Yang, X. Y. Niu, Z. X. Huang, C. H. Zhao, Y. Liu and C. Ma, Tetrahedron, 2013, 69, 8250 CrossRef CAS PubMed; (d) B. C. Yang, Z. X. Huang, H. G. Guan, X. Y. Niu, Y. Q. Li, S. Fang and C. Ma, Tetrahedron Lett., 2013, 54, 5994 CrossRef CAS PubMed; (e) X. Y. Niu, B. C. Yang, Y. Q. Li, S. Fang, Z. X. Huang, C. X. Xie and C. Ma, Org. Biomol. Chem., 2013, 11, 4102 RSC; (f) Y. L. Liu, C. J. Zhan, B. C. Yang, X. Q. Cao and C. Ma, Synlett, 2013, 111 Search PubMed; (g) Y. L. Liu, C. X. Chu, A. P. Huang, C. J. Zhan, Y. Ma and C. Ma, ACS Comb. Sci., 2011, 13, 547 CrossRef CAS PubMed; (h) S. Fang, X. Y. Niu, B. C. Yang, Y. Q. Li, X. M. Si, L. Feng and C. Ma, ACS Comb. Sci., 2014, 16, 328 CAS; (i) S. Fang, X. Y. Niu, Z. Y. Zhang, Y. Sun, X. M. Si, C. C. Shan, L. Wei, A. Q. Xu, L. Feng and C. Ma, Org. Biomol. Chem., 2014, 12, 6895 RSC.
- A. K. Verma, R. R. Jha, V. K. Sankar, T. Aggarwal, R. P. Singh and R. Chandra, Eur. J. Org. Chem., 2011, 6998 CrossRef CAS PubMed.
- Q. H. Gao, S. Liu, X. Wu and A. X. Wu, Org. Lett., 2014, 16, 4582 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qo00124b |
| This journal is © the Partner Organisations 2015 |