CuBr2-catalyzed enantioselective routes to highly functionalized and naturally occurring allenes†
Xinjun
Tang
a,
Xin
Huang
b,
Tao
Cao‡
a,
Yulin
Han‡
a,
Xingguo
Jiang‡
a,
Weilong
Lin‡
a,
Yang
Tang‡
a,
Jiasheng
Zhang‡
a,
Qiong
Yu‡
c,
Chunling
Fu
b and
Shengming
Ma
*ab
aState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, P. R. China. E-mail: masm@sioc.ac.cn
bLaboratory of Molecular Recognition and Synthesis, Department of Chemistry, Zhejiang University, Hangzhou 310027, Zhejiang, People's Republic of China
cShanghai Key Laboratory of Green Chemistry and Chemical Process, Department of Chemistry, East China Normal University, 3663 North Zhongshan Lu, Shanghai, 200062, P. R. China
First published on 9th April 2015
Abstract
Here we show the CuBr2-catalyzed approach for highly enantioselective synthesis (90–98% ee) of allenes bearing a very broad array of unmasked synthetically attractive functionalities from aldehydes and terminal alkynyl bearing reactive functionalities with the absolute configuration controlled by applying readily available (R)- or (S)-α,α-diphenylprolinol. Following this protocol, the highly enantioselective synthesis of some naturally occurring allenes loaded with reactive functionalities becomes simple: a terminal alkyne plus an aldehyde. In comparison, they were reported to be synthesized either from similar level generic chemicals with much more steps or in lower ees.
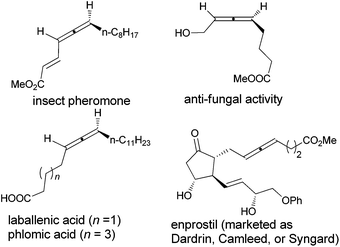
Allenes are unique unsaturated hydrocarbons in comparison with alkenes and alkynes due to the 1,2-diene-based axial chirality, which are found in over 150 biologically active natural compounds and marketed drugs (Scheme 1).1–4 The reported synthetic routes to these compounds are either with long steps or low ees.1,5
 | ||
| Scheme 1 Some typical naturally occurring optically active 1,3-disubstituted allenes and drugs with hydroxyl, carboxylic acid, and ester. | ||
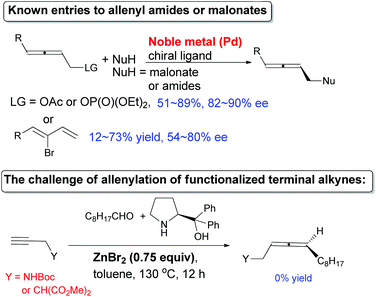
Known preparations from not-easily-available optically active propargylic derivatives and organometallic reagents, in which low efficiency of chirality transfer was often observed, led to simple non-functionalized chiral allenes with limited synthetic potentials (Fig. S1†).6 For the enantioselective synthesis of allenyl amides or malonates, the only reports are the Pd-catalyzed enantioselective reaction of racemic non-readily available 2,3-allenyl derivatives or 1,3-alkadien-3-yl bromides with moderate ees. If targeting >90% ee, one has to start with very bulky nucleophiles and substituents on the starting materials (Scheme 2, for details, see Fig. S2†).5a,7 Recently, we have developed a non-catalytic ZnX2-mediated8,9 (in some cases together with CuBr)9e allenylation reaction of terminal alkynes or propargylic ethers with aldehydes and α,α-diphenylprolinol providing entries to chiral allenes in practical ee. However, when terminal alkynyl amides or malonate were used under these reported reaction parameters, the results are rather poor (Scheme 2). Here we show that our newly developed CuBr2-catalyzed enantioselective allenylation of terminal alkynols10 may be applied to the highly efficient synthesis of some highly functionalized allenes and natural allenes.
The CuBr2-catalyzed protocol enables a one-pot synthesis from terminal propargyl amides 1a–1b to afford 2,3-allenyl tosylamine (Ra)-4aa (entry 1, Table 1) or benzamide (Ra)-4bb (entry 2, Table 1) with fairly high ees. Even the easily removable Boc group may be applied (entries 3–6, Table 1), which is vital for the introduction of other functionality to the nitrogen atom.7a–c Of course, such synthetically attractive chiral 2,3-allenyl tosylamine could also be prepared from propargyl alcohol, however, 5 steps are required.9c
| Entry | R3 | R4 | (Ra)-4 | |
|---|---|---|---|---|
| Isolated yield (%) | ee (%) | |||
| a The reaction was carried out using 1 (1.5 mmol), 2 (1.5 mmol), (S)-3 (1.0 mmol), and CuBr2 (20 mol%) in dioxane at 130 °C for 12 h unless otherwise noted. b The reaction was conducted at 70 °C for 24 h. c 40 mol% of CuBr2 was used. | ||||
| 1 | Ts (1a) | Cy (2a) | 59 ((Ra)-4aa) | 98 |
| 2 | Bz (1b) | i-Bu (2b) | 59 ((Ra)-4bb) | 93 |
| 3 | Boc (1c) | n-C8H17 (2c) | 78 ((Ra)-4cc) | 97 |
| 4b | Boc (1c) | n-C8H17 (2c) | 54 ((Ra)-4cc) | 98 |
| 5 | Boc (1c) | n-C11H23 (2d) | 74 ((Ra)-4cd) | 96 |
| 6 | Boc (1c) | Et2CH (2e) | 67 ((Ra)-4ce) | 96 |
| 7b,c | Boc (1c) | p-BrC6H4 (2f) | 60 ((Ra)-4cf) | 98 |
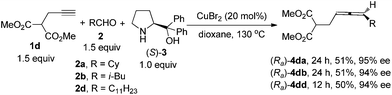
Highly optically active (2,3-butadienyl)malonates (Ra)-4da–(Ra)-4dd, which were previously prepared through 5 steps from propargyl alcohol, may also be prepared directly from dimethyl (2-propargyl)malonate 1d with one active proton remaining, which is surprising since only the terminal alkynic proton is exclusively reacted (Scheme 3).9c This also leaves opportunity for further introduction of functional groups via alkylation and synthesis of γ-allenoic acid esters due to the useful reactivity of the malonate unit.5a,7g–i
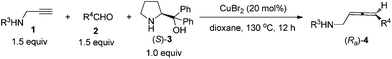
It may also be extended to simple terminal alkynes with aldehydes (Scheme 4), which has to be mediated with 50 mol% of ZnBr2 and 10–20 mol% of CuBr.9e
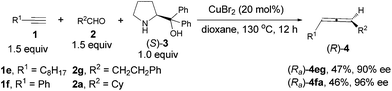
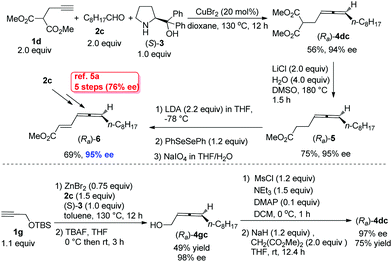
The beauty of functionality tolerance of this methodology has further been extensively demonstrated by the highly efficient enantioselective syntheses of three typical naturally occurring allenes shown in Scheme 1. In the first place, the naturally occurring insect pheromone (Ra)-6 has been synthesized from octanal in 95% ee as compared to the reported pioneering one with five steps and 76% ee from the same aldehyde via a strategy shown in Scheme 5.5a As a comparison, we have also synthesized the key intermediate (Ra)-4dc from TBS-protected propargyl alcohol 1g with three more steps including the ZnBr2-mediated construction of the allene unit,9c although the ee is slightly higher.
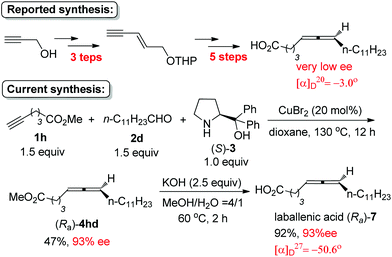
By starting from the readily available methyl 5-hexynoate, laballenic acid (Ra)-7 could be prepared in just two steps in 93% ee as compared to the enantioselective reduction-based 8-step reported synthesis from propargyl alcohol (Scheme 6).5b,11
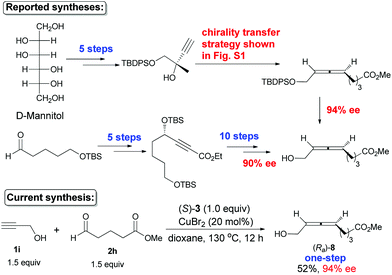
The naturally occurring 8-hydroxy-5,6-octadienoate (Ra)-8, which had been prepared with 94% ee in 7 steps from D-mannitol (based on a chirality transfer strategy)5c and 90% ee in 15 steps from 5-TBSO-substituted pentanal (via enzymatic resolution and β-elimination),5d was prepared just in one step from propargyl alcohol and 5-methoxycarbonylbutanal in 94% ee, in which both the free hydroxyl group and the ester unit survived smoothly during the transformation without a single protection–deprotection (Scheme 7).
In conclusion, the robustness of a CuBr2 catalyst for the highly enantioselective construction of 1,3-disubstituted allenes with a broad spectrum of common and synthetically versatile organic functionalities is noteworthy. Due to the excellent ees, the broad scope, and the synthetic and biological potential of these natural and unnatural chiral allenes, this versatile yet simple solution makes the synthesis of optically active 1,3-disubstituted allenes so simple (just breaking them down to the corresponding aldehydes and terminal alkynes with most of the functionalities unprotected), thus, should greatly stimulate their potential in organic chemistry and related discipline.
Acknowledgements
Financial support from the National Natural Science Foundation of China (21232006) and the National Natural Basic Research Program of China (2015CB856600) is greatly appreciated.Notes and references
- For a review on naturally occurring allenes, see: A. Hoffmann-Röder and N. Krause, Angew. Chem., Int. Ed., 2004, 43, 1196 CrossRef PubMed
.
- For selected reviews on the synthesis of allenes, see:
(a) L. K. Sydnes, Chem. Rev., 2003, 103, 1133 CrossRef CAS PubMed
; (b) N. Krause and A. Hoffmann-Röder, Tetrahedron, 2004, 60, 11671 CrossRef CAS PubMed
; (c) K. M. Brummond and J. E. De Forrest, Synthesis, 2007, 795 CrossRef CAS PubMed
; (d) M. Ogasawara, Tetrahedron: Asymmetry, 2009, 20, 259 CrossRef CAS PubMed
; (e) S. Yu and S. Ma, Chem. Commun., 2011, 47, 5384 RSC
; (f) R. K. Neff and D. E. Frantz, ACS Catal., 2014, 4, 519 CrossRef CAS
; (g) J. Ye and S. Ma, Org. Chem. Front., 2014, 1, 1210 RSC
.
- For selected recent important reports on the synthesis of chiral allenes, see:
(a) W. Zhang, H. Xu, H. Xu and W. Tang, J. Am. Chem. Soc., 2009, 131, 3832 CrossRef CAS PubMed
; (b) H. Qian, X. Yu, J. Zhang and J. Sun, J. Am. Chem. Soc., 2013, 135, 18020 CrossRef CAS PubMed
; (c) I. T. Crouch, R. K. Neff and D. E. Frantz, J. Am. Chem. Soc., 2013, 135, 4970 CrossRef CAS PubMed
; (d) T. Hashimoto, K. Sakata, F. Tamakuni, M. J. Dutton and K. Maruoka, Nat. Chem., 2013, 5, 240 CrossRef CAS PubMed
; (e) Y. Wang, W. Zhang and S. Ma, J. Am. Chem. Soc., 2013, 135, 11517 CrossRef CAS PubMed
.
- For selected reviews on the reactions of allenes, see:
(a) S. Yu and S. Ma, Angew. Chem., Int. Ed., 2012, 51, 3074 CrossRef CAS PubMed
; (b) Progress in allene chemistry, Chem. Soc. Rev., 2014, 43(9).
-
(a) M. Ogasawara, T. Nagano and T. Hayashi, J. Org. Chem., 2005, 70, 5764 CrossRef CAS PubMed
; (b) S. R. Landor, B. J. Miller and A. R. Tatchell, J. Chem. Soc. C, 1966, 1822 RSC
; (c) O. W. Gooding, C. C. Beard, D. Y. Jackson, D. L. Wren and G. F. Cooper, J. Org. Chem., 1991, 56, 1083 CrossRef CAS
; (d) Y. Zhang, H. Hao and Y. Wu, Synlett, 2010, 905 CAS
.
-
(a) J.-L. Luche, E. Barreiro, J.-M. Dollat and P. Crabbé, Tetrahedron Lett., 1975, 16, 4615 CrossRef
; (b) A. Claesson and L.-I. Olsson, Acta Chem. Scand., 1979, B33, 679 Search PubMed
; (c) C. J. Elsevier, P. Vermeer, A. Gedanken and W. Runge, Org. Chem., 1985, 50, 364 CrossRef CAS
; (d) I. Marek, P. Mangeney, A. Alexakis and J. F. Normant, Tetrahedron Lett., 1986, 27, 5499 CrossRef CAS
; (e) C. J. Elsevier and P. Vermeer, Org. Chem., 1989, 54, 3726 CrossRef CAS
; (f) A. Alexakis, I. Marek, P. Mangeney and J. F. Normant, J. Am. Chem. Soc., 1990, 112, 8042 CrossRef CAS
; (g) O. W. Gooding, C. C. Beard, D. Y. Jackson, D. L. Wren and G. F. Cooper, J. Org. Chem., 1991, 56, 1083 CrossRef CAS
; (h) P. H. Dixneuf, T. M. Guyot, D. Ness and S. M. Roberts, Chem. Commun., 1997, 2083 RSC
; (i) R. Riveiros, D. Rodríguez, J. P. Sestelo and L. A. Sarandeses, Org. Lett., 2006, 8, 1403 CrossRef CAS PubMed
; (j) M. Yoshida, T. Okada and K. Shishido, Tetrahedron, 2007, 63, 6996 CrossRef CAS PubMed
; (k) A. G. Myers and B. Zheng, J. Am. Chem. Soc., 1996, 118, 4492 CrossRef CAS
; (l) H. Ohmiya, U. Yokobori, Y. Makida and M. Sawamura, Org. Lett., 2011, 13, 6312 CrossRef CAS PubMed
; (m) M. R. Uehling, S. T. Marionni and G. Lalic, Org. Lett., 2012, 14, 362 CrossRef CAS PubMed
; (n) M. Yang, N. Yokokawa, H. Ohmiya and M. Sawamura, Org. Lett., 2012, 14, 816 CrossRef CAS PubMed
.
-
(a) B. M. Trost, D. R. Fandrick and D. C. Dinh, J. Am. Chem. Soc., 2005, 127, 14186 CrossRef CAS PubMed
; (b) Y. Imada, M. Nishida and T. Naota, Tetrahedron Lett., 2008, 49, 4915 CrossRef CAS PubMed
; (c) A. Boutier, C. Kammerer-Pentier, N. Krause, G. Prestat and G. Poli, Chem. – Eur. J., 2012, 18, 3840 CrossRef CAS PubMed
; (d) T. Nemoto, M. Kanematsu, S. Tamura and Y. Hamada, Adv. Synth. Catal., 2009, 351, 1773 CrossRef CAS PubMed
; (e) Y. Imada, M. Nishida, K. Kutsuwa, S.-I. Murahashi and T. Naota, Org. Lett., 2005, 7, 5837 CrossRef CAS PubMed
; (f) B. Wan and S. Ma, Angew. Chem., Int. Ed., 2013, 52, 441 CrossRef CAS PubMed
; (g) M. Ogasawara, H. Ikeda, T. Nagano and T. Hayashi, J. Am. Chem. Soc., 2001, 123, 2089 CrossRef CAS
; (h) M. Ogasawara, K. Ueyama, T. Nagano, Y. Mizuhata and T. Hayashi, Org. Lett., 2003, 5, 217 CrossRef CAS PubMed
; (i) M. Ogasawara, Y. Ge, A. Okada and T. Takahashi, Eur. J. Org. Chem., 2012, 1656 CrossRef CAS PubMed
.
- For a seminal paper on ZnBr2-mediated reaction of terminal alkynes, aldehydes, and amines, forming allenes, see:
(a) J. Kuang and S. Ma, J. Am. Chem. Soc., 2010, 132, 1786 CrossRef CAS PubMed
. For ZnBr2-mediated transformation of optically active propargylic amines to chiral allenes, see: (b) J. Ye, S. Li, B. Chen, W. Fan, J. Kuang, J. Liu, Y. Liu, B. Miao, B. Wan, Y. Wang, X. Xie, Q. Yu, W. Yuan and S. Ma, Org. Lett., 2012, 14, 1346 CrossRef CAS PubMed
; (c) M. Periasamy, P. O. Reddy and N. Sanjeevakumar, Tetrahedron: Asymmetry, 2014, 25, 1634 CrossRef CAS PubMed
.
- For ZnBr2- or ZnBr2-CuBr-mediated such one-pot enantioselective reactions forming chiral allenes using α,α-diphenylprolinol, see:
(a)
Ref. 8b
;
(b) M. Periasamy, N. Sanjeevakumar, M. Dalai, R. Gurubrahamam and P. O. Reddy, Org. Lett., 2012, 14, 2932 CrossRef CAS PubMed
; (c) J. Ye, W. Fan and S. Ma, Chem. – Eur. J., 2013, 19, 716 CrossRef CAS PubMed
; (d) J. Ye, R. Lü, W. Fan and S. Ma, Tetrahedron, 2013, 69, 8959 CrossRef CAS PubMed
; (e) R. Lü, J. Ye, T. Cao, B. Chen, W. Fan, W. Lin, J. Liu, H. Luo, B. Miao, S. Ni, X. Tang, N. Wang, Y. Wang, X. Xie, Q. Yu, W. Yuan, W. Zhang, C. Zhu and S. Ma, Org. Lett., 2013, 15, 2254 CrossRef PubMed
; (f) X. Zhang, Y. Qiu, C. Fu and S. Ma, Org. Chem. Front., 2014, 1, 247 RSC
.
- X. Huang, T. Cao, Y. Han, X. Jiang, W. Lin, J. Zhang and S. Ma, Chem. Commun., 2015, 51, 6956 RSC
.
- For a recent report on the synthesis of this target by another route, see: Q. Yu and S. Ma, Eur. J. Org. Chem., 2015, 1596 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section, characterization of all compounds, and copies of 1H and 13C NMR spectra of all the compounds prepared. See DOI: 10.1039/c5qo00084j |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2015 |