Enantioselective synthesis of 4,5,6,7-tetrahydroindoles via olefin cross-metathesis/intramolecular Friedel–Crafts alkylation reaction of pyrroles†
Jun-Wei
Zhang
ab,
Xiao-Wei
Liu
a,
Qing
Gu
a,
Xiao-Xin
Shi
b and
Shu-Li
You
*ab
aState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, China
bSchool of Pharmacy, East China University of Science and Technology, 130 Mei-Long Road, Shanghai 200237, China. E-mail: slyou@sioc.ac.cn
First published on 2nd March 2015
Abstract
A sequential catalysis involving olefin cross-metathesis/asymmetric intramolecular Friedel–Crafts alkylation of pyrrole derivatives has been developed. A variety of enantioenriched 4,5,6,7-tetrahydroindoles were obtained in good yields and enantioselectivity by combining a Zhan-1B catalyst with a chiral phosphoric acid.
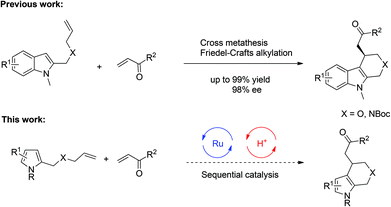
Over the past several years, sequential catalysis consisting of a transition-metal catalyst and a chiral phosphoric acid (CPA) has been one of the most effective synthetic approaches for the construction of complex molecules with diverse functional groups in a single operation.1–3 In addition, this system also features the utilization of readily available starting materials, minimization of wastes and reduction of labor. Most notably, it could achieve novel and unprecedented transformations due to the synergistic effects within two catalytic processes, providing important chiral scaffolds which could not be obtained by employing either single catalyst alone. Therefore sequential catalysis has now become an intense research area in organic synthesis. In 2009, our group demonstrated a sequential catalysis involving Ru-catalyzed olefin cross-metathesis followed by a subsequent Brønsted acid catalyzed intramolecular Friedel–Crafts alkylation of indoles, providing a variety of enantioenriched and biologically active polycyclic indoles (Scheme 1, top).4 To the best of our knowledge, however, there is no example of a sequential reaction involving cross-metathesis and Friedel–Crafts alkylation based on pyrrole scaffold despite its frequent occurrence in biologically active natural products and pharmaceuticals.5 Compared with indoles, fewer asymmetric Friedel–Crafts alkylation reactions of pyrroles6,7 were reported, likely due to the increased challenges on regio- and enantioselective control. With our continuing interest in sequential catalysis,4,8 we envisioned that cross-metathesis and asymmetric Friedel–Crafts alkylation of pyrroles might be achieved by fine tune of the substrates and catalysts (Scheme 1, below). Herein we report such a sequential catalysis involving pyrrole substrates for the synthesis of enantioenriched tetrahydroindoles.9
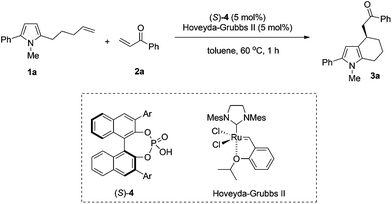
We began our studies by examining chiral phosphoric acids with different substituents in the sequential reaction between pyrrole olefin 1a and phenyl vinyl ketone 2a. The reaction of 1a and 1.5 equivalents of phenyl enone 2a in the presence of 5 mol% chiral phosphoric acid (S)-4 and 5 mol% Hoveyda–Grubbs II in toluene at 60 °C all proceeded to completion within 1 hour to give the desired product in good yields (56–71%) and moderate to good enantioselectivity (18–62% ee). As summarized in Table 1, the substituent of the catalyst had a great influence on the enantioselectivity of the reaction. Chiral phosphoric acids 4c bearing 1-naphthyl groups and 4e bearing 4-biphenyl groups proved to be the most efficient catalysts in terms of reactivity and enantioselectivity, affording 3a in 56% yield, 62% ee and 71% yield, 60% ee respectively (Table 1, entries 3 and 5).
| Entry | 4, Ar | Yieldb (%) | eec (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.20 mmol), 2a (0.30 mmol), Hoveyda–Grubbs II (5 mol%) and (S)-4 (5 mol%) in toluene (2 mL) at 60 °C. b Isolated yield. c Determined by HPLC analysis. | |||
| 1 | 4a, 2,4,6-(iPr)3-C6H2 | 61 | 18 |
| 2 | 4b, SiPh3 | 65 | 20 |
| 3 | 4c, 1-naphthyl | 56 | 62 |
| 4 | 4d, 2-naphthyl | 64 | 19 |
| 5 | 4e, 4-biphenyl | 71 | 60 |
| 6 | 4f, 4-NO2-C6H4 | 67 | 44 |
| 7 | 4g, 4-[3,5-(CF3)2-C6H3]-C6H4 | 66 | 50 |
| 8 | 4h, 9-anthryl | 60 | 52 |
| 9 | 4i, 9-phenanthryl | 59 | 55 |
| 10 | 4j, 3,5-(CF3)2-C6H3 | 61 | 29 |
| 11 | 4k, 2-isopropoxy-1-naphthyl | 66 | 54 |
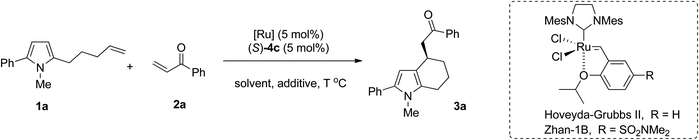
Encouraged by these results, other reaction parameters such as reaction temperature, ruthenium catalysts and solvents were further investigated with (S)-4c as the optimal Brønsted acid. The results are summarized in Table 2. At a lower temperature, the reaction delivered the corresponding product with an increased ee value albeit slightly decreased yield (Table 2, entries 1–4). For example, when the reaction was performed at 40 °C, the ee value of the product could be increased to 72% (Table 2, entry 2). Notably, Zhan-1B could also provide product 3a in 70% ee with a slightly higher yield (54% yield) (Table 2, entry 5). Given its cheapness, Zhan-1B was used for further optimization of the reaction conditions. Among the molecular sieves with different sizes, the addition of 3 Å MS gave better results (Table 2, entry 6, 60% yield, 72% ee). Other solvents such as o-xylene, CH2Cl2, THF and ether all led to the formation of 3a in comparable yields but with decreased enantioselectivity (Table 2, entries 9–12, 66–72% yields and 32–54% ee). To our great delight, the amount of enone 2a could be further reduced to 1.2 equivalents without the erosion of yield and enantioselectivity (Table 2, entry 13, 60% yield, 72% ee). Thus the optimized conditions were obtained as the following: 5 mol% of (S)-4c, 5 mol% of Zhan-1B, 1.2 equivalents of enone 2, 3 Å MS as an additive in toluene at 40 °C.
| Entry | [Ru] | Additive | Solvent | Temp (°C) | Time (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.20 mmol), 2a (0.30 mmol), Ru catalyst (5 mol%), (S)-4c (5 mol%) and MS (100 mg) in solvent (2 mL). b Isolated yield. c Determined by HPLC analysis. d 0.24 mmol of 2a was used. | |||||||
| 1 | Hoveyda–Grubbs II | None | Toluene | 60 | 1 | 56 | 62 |
| 2 | Hoveyda–Grubbs II | None | Toluene | 40 | 1 | 50 | 72 |
| 3 | Hoveyda–Grubbs II | None | Toluene | rt | 10 | 52 | 70 |
| 4 | Hoveyda–Grubbs II | None | Toluene | 0 | 10 | 25 | 68 |
| 5 | Zhan-1B | None | Toluene | 40 | 1 | 54 | 70 |
| 6 | Zhan-1B | 3 Å MS | Toluene | 40 | 1 | 60 | 72 |
| 7 | Zhan-1B | 4 Å MS | Toluene | 40 | 1 | 58 | 70 |
| 8 | Zhan-1B | 5 Å MS | Toluene | 40 | 1 | 43 | 72 |
| 9 | Zhan-1B | 3 Å MS | CH2Cl2 | 40 | 1.5 | 66 | 50 |
| 10 | Zhan-1B | 3 Å MS | o-Xylene | 40 | 1 | 67 | 44 |
| 11 | Zhan-1B | 3 Å MS | Et2O | 40 | 3 | 66 | 54 |
| 12 | Zhan-1B | 3 Å MS | THF | 40 | 1 | 72 | 32 |
| 13 | Zhan-1B | 3 Å MS | Toluene | 40 | 1 | 60 | 72 |
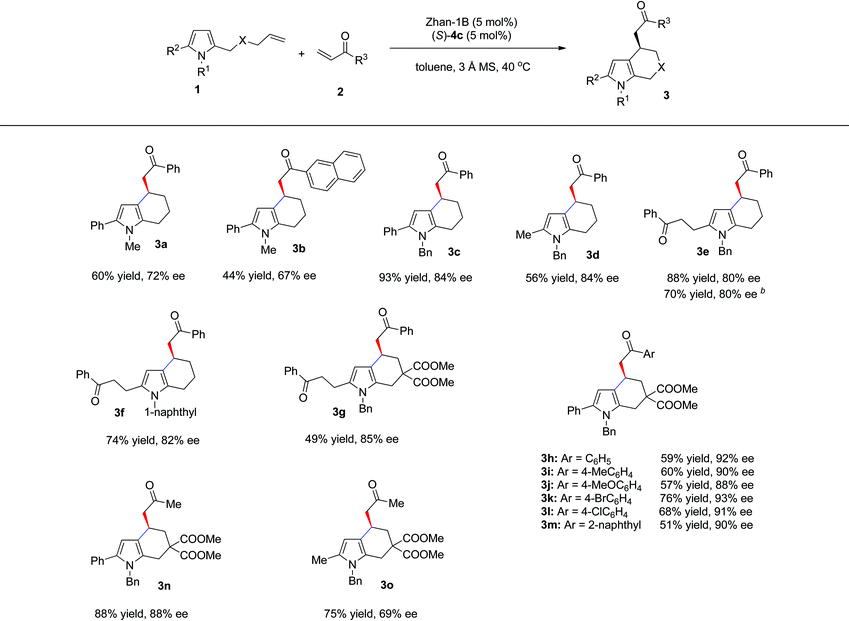
Under the above mentioned optimized reaction conditions, we then examined the substrate scope of this reaction. The results are summarized in Table 3. Besides phenyl enone, 2-naphthyl enone (2b) was also a suitable substrate, giving 3b in 44% yield and 67% ee. With N-Bn protected pyrrole olefin (2c) as a substrate, both enantioselectivity and yield were significantly increased (Table 3, 3c, 93% yield, 84% ee). When the phenyl group at the C5 position of pyrrole was replaced by methyl or benzoylethyl, the sequential reaction also occurred smoothly, affording the tetrahydroindole products in good yields and ee (3d: 56% yield, 84% ee; 3e: 88% yield, 80% ee). Interestingly, when pyrrole olefin without a substituent at the C5 position (1c, R1 = Bn, R2 = H, X = CH2) was used, with 2.0 equivalents of enone 2a, the same product (3e) could be obtained with identical ee and slightly decreased yield (70% yield, 80% ee) through an intermolecular Friedel–Crafts, cross-metathesis and intramolecular Friedel–Crafts reaction cascade. In addition, N-1-naphthyl protected pyrrole gave comparable results (3f: 74% yield, 82% ee). The carbon-tethered pyrrole olefin was also compatible under the optimal reaction conditions, affording 3g in 49% yield and 85% ee. Other substituted pyrrole olefins bearing an electron-donating or electron-withdrawing group were also well tolerated and led to their corresponding products (3h–m) in good yields (51–76%) and excellent enantioselectivity (88–93% ee). It is worth noting that aliphatic enone such as methyl vinyl ketone was also a suitable substrate (3n, 88% yield, 88% ee; 3o, 75% yield, 69% ee).
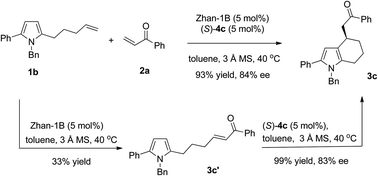
The advantage of a sequential reaction was demonstrated by comparison with the synthesis of tetrahydroindole 3c in a stepwise approach. The olefin cross-metathesis reaction of pyrrole olefin 1b with phenyl vinyl ketone 2a catalyzed by Zhan-1B gave intermediate 3c′ in 33% yield together with racemic 3c in 52% yield due to its easy cyclization during purification. Tetrahydroindole 3c was then obtained through (S)-4c catalyzed intramolecular Friedel–Crafts alkylation in 33% yield and 83% ee over two steps (Scheme 2). The sequential reaction avoids troublesome separation processes and increases the yield of the synthesis dramatically.
In summary, we have developed Zhan-1B in combination with a chiral phosphoric acid catalyzed olefin cross-metathesis/asymmetric intramolecular Friedel–Crafts alkylation of pyrrole derivatives. This sequential catalysis provides a concise and efficient approach to construct chiral 4,5,6,7-tetrahydroindoles in good yields and enantioselectivity from readily available starting materials. Development of more sequential reactions based on dual catalysis is currently ongoing in our laboratory.
Acknowledgements
We thank the National Basic Research Program of China (973 Program 2015CB856600) and the National Natural Science Foundation of China (21272253, 21332009, 21361140373, 21421091) for generous financial support.Notes and references
- For reviews on the combination of a transition metal and chiral phosphoric acid catalysis: (a) Z. Shao and H. Zhang, Chem. Soc. Rev., 2009, 38, 2745 RSC; (b) M. Rueping, R. M. Koenigs and I. Atodiresei, Chem. – Eur. J., 2010, 16, 9350 CrossRef CAS PubMed; (c) J. Zhou, Chem. – Asian J., 2010, 5, 422 CrossRef CAS PubMed; (d) C. Zhong and X. Shi, Eur. J. Org. Chem., 2010, 2999 CrossRef CAS; (e) Z. Du and Z. Shao, Chem. Soc. Rev., 2013, 42, 1337 RSC; (f) X. Wu, M. Li and L. Gong, Acta Chim. Sin., 2013, 71, 1091 CrossRef CAS; (g) F. Lv, S. Liu and W. Hu, Asian J. Org. Chem., 2013, 2, 824 CrossRef CAS; (h) D. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047 CrossRef CAS PubMed; (i) D.-F. Chen, Z.-Y. Han, X.-L. Zhou and L.-Z. Gong, Acc. Chem. Res., 2014, 47, 2365 CrossRef CAS PubMed; (j) Z.-P. Yang, W. Zhang and S.-L. You, J. Org. Chem., 2014, 79, 7785 CrossRef CAS PubMed.
- Selected examples of the combination of a transition metal and chiral phosphoric acid catalysis: (a) K. Sorimachi and M. Terada, J. Am. Chem. Soc., 2008, 130, 14452 CrossRef CAS PubMed; (b) W. Hu, X. Xu, J. Zhou, W.-J. Liu, H. Huang, J. Hu, L. Yang and L.-Z. Gong, J. Am. Chem. Soc., 2008, 130, 7782 CrossRef CAS PubMed; (c) Z.-Y. Han, H. Xiao, X.-H. Chen and L.-Z. Gong, J. Am. Chem. Soc., 2009, 131, 9182 CrossRef CAS PubMed; (d) M. Terada and Y. Toda, J. Am. Chem. Soc., 2009, 131, 6354 CrossRef CAS PubMed; (e) M. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt and D. J. Dixon, J. Am. Chem. Soc., 2009, 131, 10796 CrossRef CAS PubMed; (f) B. Xu, S.-F. Zhu, X.-L. Xie, J.-J. Shen and Q.-L. Zhou, Angew. Chem., Int. Ed., 2011, 50, 11483 CrossRef CAS PubMed; (g) J. Jiang, H.-D. Xu, J.-B. Xi, B.-Y. Ren, F.-P. Lv, X. Guo, L.-Q. Jiang, Z.-Y. Zhang and W.-H. Hu, J. Am. Chem. Soc., 2011, 133, 8428 CrossRef CAS PubMed; (h) Q.-A. Chen, D.-S. Wang, Y.-G. Zhou, Y. Duan, H.-J. Fan, Y. Yang and Z. Zhang, J. Am. Chem. Soc., 2011, 133, 6126 CrossRef CAS PubMed; (i) Q.-A. Chen, M.-W. Chen, C.-B. Yu, L. Shi, D.-S. Wang, Y. Yang and Y.-G. Zhou, J. Am. Chem. Soc., 2011, 133, 16432 CrossRef CAS PubMed; (j) E. Ascic, J. F. Jensen and T. E. Nielsen, Angew. Chem., Int. Ed., 2011, 50, 5188 CrossRef CAS PubMed; (k) E. Ascic, C. L. Hansen, S. T. Le Quement and T. E. Nielsen, Chem. Commun., 2012, 48, 3345 RSC; (l) Z.-Y. Han, D.-F. Chen, Y.-Y. Wang, R. Guo, P.-S. Wang, C. Wang and L.-Z. Gong, J. Am. Chem. Soc., 2012, 134, 6532 CrossRef CAS PubMed; (m) X.-F. Tu and L.-Z. Gong, Angew. Chem., Int. Ed., 2012, 51, 11346 CrossRef CAS PubMed; (n) M. Terada and Y. Toda, Angew. Chem., Int. Ed., 2012, 51, 2093 CrossRef CAS PubMed; (o) H. Qiu, M. Li, L.-Q. Jiang, F.-P. Lv, L. Zan, C.-W. Zhai, M. P. Doyle and W.-H. Hu, Nat. Chem., 2012, 4, 733 CrossRef CAS PubMed; (p) Q.-A. Chen, K. Gao, Y. Duan, Z.-S. Ye, L. Shi, Y. Yang and Y.-G. Zhou, J. Am. Chem. Soc., 2012, 134, 2442 CrossRef CAS PubMed; (q) C. L. Hansen, J. W. Clausen, R. G. Ohm, E. Ascic, S. T. Le Quement, D. Tanner and T. E. Nielsen, J. Org. Chem., 2013, 78, 12545 CrossRef CAS PubMed; (r) H. Wu, Y.-P. He and L.-Z. Gong, Org. Lett., 2013, 15, 460 CrossRef CAS PubMed; (s) H. Liu, C. Zeng, J. Guo, M. Zhang and S. Yu, RSC Adv., 2013, 3, 1666 RSC; (t) V. M. Lombardo, C. D. Thomas and K. A. Scheidt, Angew. Chem., Int. Ed., 2013, 52, 12910 CrossRef CAS PubMed; (u) A. W. Gregory, P. Jakubec, P. Turner and D. J. Dixon, Org. Lett., 2013, 15, 4330 CrossRef CAS PubMed; (v) B. Xu, S.-F. Zhu, X.-D. Zuo, Z.-C. Zhang and Q.-L. Zhou, Angew. Chem., Int. Ed., 2014, 53, 3913 CrossRef CAS PubMed; (w) Z.-P. Chen, M.-W. Chen, R.-N. Guo and Y.-G. Zhou, Org. Lett., 2014, 16, 1406 CrossRef CAS PubMed.
- Pioneering works on chiral phosphoric acid, see: (a) D. Uraguchi, K. Sorimachi and M. Terada, J. Am. Chem. Soc., 2004, 126, 11804 CrossRef CAS PubMed; (b) T. Akiyama, J. Itoh, K. Yokota and K. Fuchibe, Angew. Chem., Int. Ed., 2004, 43, 1566 CrossRef CAS PubMed. For selected reviews on chiral phosphoric acid, see: (c) T. Akiyama, Chem. Rev., 2007, 107, 5744 CrossRef CAS PubMed; (d) Y.-J. Gao, L.-H. Yang, S.-J. Song, J.-J. Ma, R.-X. Tang, R.-H. Bian, H.-Y. Liu, Q.-H. Wu and C. Wang, Chin. J. Org. Chem., 2008, 28, 8 CAS; (e) Y. Su and F. Shi, Chin. J. Org. Chem., 2010, 30, 486 CAS; (f) M. Terada, Chem. Commun., 2008, 4097 RSC; (g) M. Terada, Synthesis, 2010, 1929 CrossRef CAS PubMed; (h) A. Zamfir, S. Schenker, M. Freund and S. B. Tsogoeva, Org. Biomol. Chem., 2010, 8, 5262 CAS; (i) M. Rueping, A. Kuenkel and I. Atodiresei, Chem. Soc. Rev., 2011, 40, 4539 RSC.
- (a) Q. Cai, Z.-A. Zhao and S.-L. You, Angew. Chem., Int. Ed., 2009, 48, 7428 CrossRef CAS PubMed. For other works on sequential catalysis of the cross-metathesis/Friedel–Crafts alkylation reaction see: (b) J.-R. Chen, C.-F. Li, X.-L. An, J.-J. Zhang, X.-Y. Zhu and W.-J. Xiao, Angew. Chem., Int. Ed., 2008, 47, 2489 CrossRef CAS PubMed; (c) X.-L. An, J.-R. Chen, C.-F. Li, F.-G. Zhang, Y.-Q. Zou, Y.-C. Guo and W.-J. Xiao, Chem. – Asian J., 2010, 5, 2258 CrossRef CAS PubMed.
- For reviews: (a) A. Fürstner, Angew. Chem., Int. Ed., 2003, 42, 3582 CrossRef PubMed; (b) G. Balme, Angew. Chem., Int. Ed., 2004, 43, 6238 CrossRef CAS PubMed; (c) H. Fan, J. Peng, M. T. Hamann and J. F. Hu, Chem. Rev., 2008, 108, 264 CrossRef CAS PubMed; (d) S. Thirumalairajan, B. M. Pearce and A. Thompson, Chem. Commun., 2010, 46, 1797 RSC; (e) R. Rane, N. Sahu, C. Shah and R. Karpoormath, Curr. Top. Med. Chem., 2014, 14, 253 CrossRef CAS; (f) A. Fürstner, K. Radkowski and H. Peters, Angew. Chem., Int. Ed., 2005, 44, 2777 CrossRef PubMed; (g) P. A. Duspara and R. A. Batey, Angew. Chem., Int. Ed., 2013, 52, 10862 CrossRef CAS PubMed.
- For a book, see: (a) Catalytic Asymmetric Friedel–Crafts Alkylations, ed. M. Bandini and A. Umani-Ronchi, Wiley-VCH, Weinheim, 2009 Search PubMed. For reviews on an asymmetric Friedel–Crafts reaction, see: (b) Y. Wang and K.-L. Ding, Chin. J. Org. Chem., 2001, 21, 763 CAS; (c) M. Bandini, A. Melloni and A. Umani-Ronchi, Angew. Chem., Int. Ed., 2004, 43, 550 CrossRef CAS PubMed; (d) M. Bandini, A. Melloni, S. Tommasi and A. Umani-Ronchi, Synlett, 2005, 1199 CrossRef CAS PubMed; (e) Y.-F. Sheng, A. J. Zhang, X.-J. Zheng and S.-L. You, Chin. J. Org. Chem., 2008, 28, 605 CAS; (f) T. B. Poulsen and K. A. Jørgensen, Chem. Rev., 2008, 108, 2903 CrossRef CAS PubMed; (g) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190 RSC; (h) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS PubMed; (i) M. Zeng and S.-L. You, Synlett, 2010, 1289 CAS; (j) V. Terrasson, R. M. de Figueiredo and J. M. Campagne, Eur. J. Org. Chem., 2010, 2635 CrossRef CAS; (k) M. Rueping and B. J. Nachtsheim, Beilstein J. Org. Chem., 2010, 6, 6 Search PubMed; (l) P. Chauhan and S. S. Chimni, RSC Adv., 2012, 2, 6117 RSC; (m) S. Lancianesi, A. Palmieri and M. Petrini, Chem. Rev., 2014, 114, 7108 CrossRef CAS PubMed.
- For a pioneering asymmetric Friedel–Crafts alkylation of pyrrole, see: (a) N. A. Paras and D. W. C. MacMillan, J. Am. Chem. Soc., 2001, 123, 4370 CrossRef CAS. Selected latest examples, see: (b) Y.-F. Sheng, Q. Gu, A.-J. Zhang and S.-L. You, J. Org. Chem., 2009, 74, 6899 CrossRef CAS PubMed; (c) P. K. Singh and V. K. Singh, Org. Lett., 2010, 12, 80 CrossRef CAS PubMed; (d) W. Kashikura, J. Itoh, K. Mori and T. Akiyama, Chem. – Asian J., 2010, 5, 470 CrossRef CAS PubMed; (e) F. Guo, D. Chang, G. Lai, T. Zhu, S. Xiong, S. Wang and Z. Wang, Chem. – Eur. J., 2011, 17, 11127 CrossRef CAS PubMed; (f) K. Aikawa, K. Honda, S. Mimura and K. Mikami, Tetrahedron Lett., 2011, 52, 6682 CrossRef CAS PubMed; (g) L. Liu, H. Ma, Y. Xiao, F. Du, Z. Qin, N. Li and B. Fu, Chem. Commun., 2012, 48, 9281 RSC; (h) M. Zeng, W. Zhang and S.-L. You, Chin. J. Chem., 2012, 30, 2615 CrossRef CAS; (i) D. Hack and D. Enders, Synthesis, 2013, 2904 CAS; (j) D. Zhang, H. Qiu, L. Jiang, F. Lv, C. Ma and W. Hu, Angew. Chem., Int. Ed., 2013, 52, 13356 CrossRef CAS PubMed; (k) Y.-Z. Hua, X.-W. Han, X.-C. Yang, X. Song, M.-C. Wang and J.-B. Chang, J. Org. Chem., 2014, 79, 11690 CrossRef CAS PubMed.
- (a) Q. Cai, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2010, 49, 8666 CrossRef CAS PubMed; (b) Q. Cai, X.-W. Liang, S.-G. Wang, J.-W. Zhang, X. Zhang and S.-L. You, Org. Lett., 2012, 14, 5022 CrossRef CAS PubMed; (c) J.-W. Zhang, Z. Xu, Q. Gu, X.-X. Shi, X.-B. Leng and S.-L. You, Tetrahedron, 2012, 68, 5263 CrossRef CAS PubMed; (d) Q. Cai, X.-W. Liang, S.-G. Wang and S.-L. You, Org. Biomol. Chem., 2013, 11, 1602 RSC; (e) J.-W. Zhang, Q. Cai, Q. Gu, X.-X. Shi and S.-L. You, Chem. Commun., 2013, 49, 7750 RSC; (f) Y.-C. Shi, S.-G. Wang, Q. Yin and S.-L. You, Org. Chem. Front., 2014, 1, 39 RSC.
- J. C. Conrad, J. Kong, B. N. Laforteza and D. W. C. MacMillan, J. Am. Chem. Soc., 2009, 131, 11640 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1045810. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00034c |
| This journal is © the Partner Organisations 2015 |