One pot cascade synthesis of fused heterocycles from furan-tethered terminal alkynes and aldehydes in the presence of amines and CuBr†
Yan-Yan
Zhang
a,
Jian
Hao
*a and
Min
Shi
*b
aDepartment of Chemistry, Shanghai University, 99 Shangda Road, Shanghai 200444, China
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Lu, Shanghai 200032, China. E-mail: mshi@mail.sioc.ac.cn; Fax: +86-21-64166128
First published on 12th February 2015
Abstract
A novel one-pot protocol for the construction of complex heterocycles through furan tethered terminal alkynes, aldehydes, amines and CuBr upon heating has been developed, giving the cycloadducts in moderate to high yields along with moderate to good regioselectivities. The reactions proceed through a two-component domino reaction including allenation and subsequent intramolecular Diels–Alder reaction. A wide range of aliphatic or aromatic aldehydes and furan tethered terminal alkynes are well-tolerated, enriching the chemistry of the intramolecular Diels–Alder reaction related to furan.
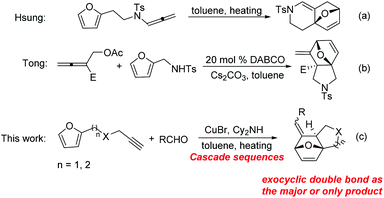
The intramolecular Diels–Alder reaction related to furan (IMDAF) is one of the most widely utilized reactions for the construction of heterocyclic ring systems because furan is an excellent diene in Diels–Alder reactions.1,2 On the other hand, allenes have been widely used as dienophiles in Diels–Alder reaction upon heating or in the presence of metal catalysts.3 Among the intramolecular cycloaddition reactions between furan and an allene moiety, two modes are mainly involved as shown in Scheme 1. The first one is the cycloaddition of furan with the distal double bond of the allene, giving rise to a bridged bicyclic skeleton containing an endocyclic double bond. The second one is the cycloaddition of furan with the proximal double bond of the allene, affording a bridged bicyclic skeleton bearing an exocyclic double bond.1f
Due to the importance of complex aza-heterocycles, recently, an array of transition-metal-catalyzed cycloaddition reactions of dienes with allenes have been reported.4 Paralleling with metal-catalyzed ones, thermal and DABCO (1,4-diazabicyclo[2,2,2]octane) catalyzed cycloaddition have also been developed to construct these scaffolds. For example, Hsung and coworkers have reported a thermal intramolecular [4 + 2] cycloaddition of furan tethered allenamides without the assistance of any metal catalyst for rapid assembly of highly functionalized nitrogen heterocycles (reaction (a), Scheme 1).5 In addition, Tong et al. revealed the DABCO-triggered cascade SN2/cycloaddition sequence between 2-(acetoxymethyl)buta-2,3-dienoate and various π-system functionalized tosylamides to construct structurally diverse aza-heterocycles (reaction (b), Scheme 1).6
Inspired by these findings, herein, we wish to report a one-pot protocol for the synthesis of fused heterocycles through furan tethered allenes via a two-component reaction by the use of aldehydes, furan tethered terminal alkynes, and dicyclohexamine as the reactants (reaction (c), Scheme 1). This one-pot manner featured a powerful synthetic approach to the construction of fused heterocycles with increased molecular diversity and complexity.7
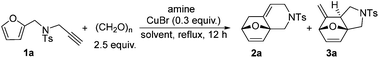
Initially, we attempted to in situ synthesize furan tethered allene using alkyne 1a, paraformaldehyde and amine in 1,4-dioxane according to Ma's procedures and subsequently to explore the next sequential IMDAF.8 Therefore, we first examined the reaction outcome using N-(furan-2-ylmethyl)-4-methyl-N-(prop-2-yn-1-yl)benzenesulfonamide 1a as the substrate, and the results are shown in Table 1. Under the reported standard conditions, the reaction of 1a with paraformaldehyde (2.5 equiv.), i-Pr2NH (1.8 equiv.) and CuBr (0.3 equiv.) in 1,4-dioxane afforded the desired cycloadducts 2a and 3a in 36% total yield as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 regioisomers (Table 1, entry 1). Their structures were confirmed by X-ray diffraction (Fig. 1) and the related CIF data are shown in the ESI.†
3 regioisomers (Table 1, entry 1). Their structures were confirmed by X-ray diffraction (Fig. 1) and the related CIF data are shown in the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9,10 The structures of 2a and 3a clearly indicated that the subsequent IMDAF indeed took place with the distal and proximal double bond of the allene moiety to give the fused heterocycles bearing an endocyclic and exocyclic double bond, respectively (Fig. 1).
9,10 The structures of 2a and 3a clearly indicated that the subsequent IMDAF indeed took place with the distal and proximal double bond of the allene moiety to give the fused heterocycles bearing an endocyclic and exocyclic double bond, respectively (Fig. 1).
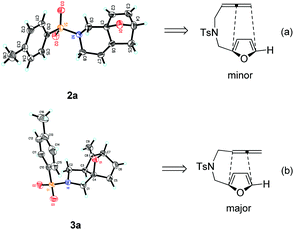 | ||
| Fig. 1 X-ray crystal structures of 2a (a) and 3a (b) and the regioselectivity for [4 + 2] cycloaddition. | ||
| Entrya | Amines (equiv.) | Solvents | Yieldb (%) |
|---|---|---|---|
| 2a + 3a (ratio)c | |||
| a The reactions were carried out on a 0.2 mmol scale in 1 mL of solvents. b Determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard. c The value in the parentheses is the ratio of isomers 2a and 3a that was determined by 1H NMR spectroscopy. d Isolated yields. e The reaction was carried out at 80 °C. f 2.0 equiv. (CH2O)n was added. g 0.20 equiv. CuBr was added. | |||
| 1 | i-Pr2NH (1.8) | Dioxane | 36 (1/3) |
| 2 |
|
Dioxane | 22 (1/4) |
| 3 | Cy2NH (1.8) | Dioxane | 78 (1/4) |
| 4 | i-Pr2NH (1.8) | Toluene | 51 (1/4) |
| 5 | Cy2NH (1.8) | Toluene | 86 (1/4)d |
| 6e | Cy2NH (1.8) | Toluene | 21 (1/4) |
| 7f | Cy2NH (1.8) | Toluene | 67 (1/4) |
| 8g | Cy2NH (1.8) | Toluene | 67 (1/4) |
| 9 | Cy2NH (1.5) | Toluene | 77 (1/4) |
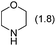
Next, by screening different amines, we found that the total yield of 2a and 3a was improved to 78% when dicyclohexylamine was employed as the base (Table 1, entries 2 and 3). Further investigations led to the observation that when toluene was used as the solvent, the corresponding products 2a and 3a could be isolated in 86% total yield (Table 1, entry 5). The examinations on the amounts of dicyclohexylamine, paraformaldehyde and CuBr as well as the reaction temperature revealed that the use of 1.8 equiv. of amine, 2.5 equiv. of aldehyde and 0.3 equiv. of CuBr under reflux in toluene gave the best performance (Table 1, entries 6–9).
With the optimized conditions in hand, we next sought to determine the scope of alkynes that can be employed in this new tandem cycloaddition protocol. As shown in Table 2, changing the substituent on the nitrogen atom from Ts to Bs or Ns (Bs = p-bromobenzenesulfonyl, Ns = 4-nitrobenzenesulfonyl) did not significantly affect the reaction outcome, giving both regioisomers 2 and 3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
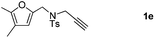
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio) in 63–80% total yields (Table 2, entries 2 and 3). Moreover, no 3a could be detected upon heating 2a in toluene at 110 °C for 48 h on the basis of 1H NMR spectroscopy. This result indicated that 2a cannot be transformed into 3avia the retro-cycloaddition and cycloaddition process. Substrates 1d–1e with different furan substitutions produced the corresponding cycloadducts 2 and 3 (1
5 ratio) in 63–80% total yields (Table 2, entries 2 and 3). Moreover, no 3a could be detected upon heating 2a in toluene at 110 °C for 48 h on the basis of 1H NMR spectroscopy. This result indicated that 2a cannot be transformed into 3avia the retro-cycloaddition and cycloaddition process. Substrates 1d–1e with different furan substitutions produced the corresponding cycloadducts 2 and 3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
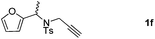
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) in 60% total yields (Table 2, entries 4 and 5). Notably, as for substrate 1f having a methyl substituent at the carbon tether, the reaction also proceeded smoothly to give the desired products 2f and 3f in 81% total yield as 1
4 ratio) in 60% total yields (Table 2, entries 4 and 5). Notably, as for substrate 1f having a methyl substituent at the carbon tether, the reaction also proceeded smoothly to give the desired products 2f and 3f in 81% total yield as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
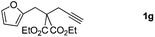
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 regioisomers (Table 2, entry 6). Furthermore, substrate 1g with a C(CO2Et)2 carbon linkage was also found to be suitable in this reaction, furnishing the desired product 3g in 46% yield as a single regioisomer (Table 2, entry 7). Substrate 1h connected by an oxygen atom was also tolerated in this protocol, albeit affording 3h in 14% yield (Table 2, entry 8).11
2 regioisomers (Table 2, entry 6). Furthermore, substrate 1g with a C(CO2Et)2 carbon linkage was also found to be suitable in this reaction, furnishing the desired product 3g in 46% yield as a single regioisomer (Table 2, entry 7). Substrate 1h connected by an oxygen atom was also tolerated in this protocol, albeit affording 3h in 14% yield (Table 2, entry 8).11
| Entrya | Substrate | Yieldb (%) |
|---|---|---|
| 2 + 3 (ratio)c | ||
| a The reactions were carried out on a 0.2 mmol scale in 1.0 mL of toluene. b Isolated yields. c The value in the parentheses is the ratio of isomers 2 and 3 that was determined by 1H NMR spectroscopy. d 2f was found as a ∼3/1 inseparable isomeric mixture and 3f was found as a ∼2/1 inseparable isomeric mixture. e Only 3g was isolated. f Only 3h was isolated. | ||
| 1 |
|
86 (1/4) |
| 2 | 63 (1/4) | |
| 3 | 80 (1/5) | |
| 4 |
|
60 (1/1) |
| 5 |
|
60 (1/4) |
| 6 |
|
81 (1/2)d |
| 7e |
|
46 |
| 8f |

|
14 |
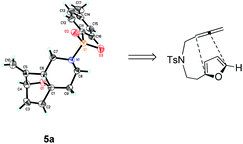
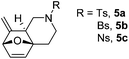
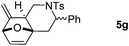
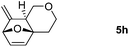
Interestingly, we found that substrate 4a having a CH2CH2 carbon tether at the furan moiety gave the corresponding cycloadduct 5a as a single regioisomer in 85% yield (Table 3, entry 1), suggesting that extending one carbon atom at the tether provides a sterically demanding cycloadduct in favour of cycloaddition for the furan with the proximal double bond of allene moiety (Fig. 2). Its structure has been confirmed by X-ray diffraction (Fig. 2) and the related CIF data are shown in the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12
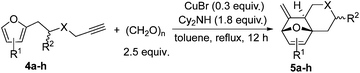
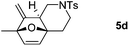
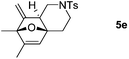
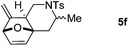
We next attempted to determine the scope of substrates 4 that can be employed in this one-pot protocol. As shown in Table 3, the substrate scope similarly consists of (1) different N-substituents (Table 3, entries 1–3); (2) various furan substitutions (Table 3, entries 4 and 5); (3) the substrates 4f and 4g having a methyl group or phenyl group at the carbon tether. All these reactions proceeded efficiently, affording the corresponding products 5b–5g in 56–87% yields as a single regioisomer or 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–7
1–7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoisomers, respectively (Table 3, entries 2–7). Substrate 4h tethered by an oxygen atom afforded the corresponding product 5h in 9% yield (Table 3, entry 8).
1 diastereoisomers, respectively (Table 3, entries 2–7). Substrate 4h tethered by an oxygen atom afforded the corresponding product 5h in 9% yield (Table 3, entry 8).
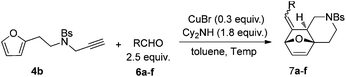
To further illustrate the substrate scope, a variety of other aldehydes 6 have been examined in this cascade reaction under the standard conditions and the results are summarized in Table 4.
| Entry | R | Temp. (°C) | Time (h) | Yielda (%) | Ratio (E/Z) | |
|---|---|---|---|---|---|---|
| a Isolated yield. b The reaction was carried out using 1,2,3,4-tetrahydroisoquinoline replacing dicyclohexylamine. c The reactions were carried out in a sealed tube. d Inseparable isomeric mixture. | ||||||
| 1b | Ph | 6a | 110 | 7 | 55 | E |
| 2 | p-ClPh | 6b | 110 | 14 | 47 | E |
| 3 |
|
6c | 110 | 19 | 32 | E |
| 4c,d | CH3(CH2)3 | 6d | 130 | 24 | 37 | 10/1 |
| 5c,d | CH3(CH2)4 | 6e | 150 | 12 | 43 | 9/1 |
| 6c,d | (CH3)2CH | 6f | 150 | 11 | 33 | 25/1 |
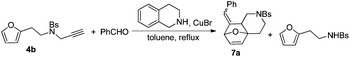
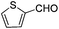
Substrate 4b reacted with benzaldehyde 6a or p-chlorobenzaldehyde 6b smoothly using tetrahydroisoquinoline or dicyclohexylamine as the base respectively,13,14 affording the corresponding cycloadducts 7a and 7b in moderate yields as a single isomer with E-configuration on the basis of NOESY spectra (Table 4, entries 1 and 2, also see ESI†). A heteroaromatic aldehyde such as 2-thenaldehyde 6c was also suitable in this transformation (Table 4, entry 3). Moreover, aliphatic aldehydes 6d–6f have been also tested. It was realized that high reaction temperature and longer reaction time are required, giving the desired products 7d–7f in 33–43% yields mainly as E-configuration, suggesting a wide substrate range of this domino sequence (Table 4, entries 4–6).
In conclusion, we have disclosed a novel synthetic protocol for the construction of complex heterocycles through an efficient one-pot reaction of furan tethered alkynes and aldehydes with amines catalyzed by CuBr via a cascade two-component condensation and the subsequent intramolecular Diels–Alder reaction related to furan (IMDAF), providing the desired products in moderate to high yields along with moderate to good regioselectivities. A wide range of aliphatic or aromatic aldehydes and furan tethered terminal alkynes are tolerated. Further investigations on expanding the scope of this reaction towards a wide range of other alkynes as well as the applications of this protocol to natural product synthesis are in progress.
Acknowledgements
We are grateful for the financial support from the National Basic Research Program of China (973)-2015CB856603, Shanghai Municipal Committee of Science and Technology (11JC1402600), the National Natural Science Foundation of China (20472096, 21372241, 21361140350, 20672127, 21421091, 21372250, 21121062, 21302203 20732008, and 21172141).Notes and references
- For selected examples, see: (a) W. Oppolzer, Angew. Chem., Int. Ed. Engl., 1977, 16, 10 CrossRef; (b) G. Brieger and J. N. Bennett, Chem. Rev., 1980, 80, 63 CrossRef CAS; (c) A. G. Fallis, Can. J. Chem., 1984, 62, 183 CrossRef CAS; (d) D. Craig, Chem. Soc. Rev., 1987, 16, 187 RSC; (e) A. G. Fallis, Acc. Chem. Res., 1999, 32, 464 CrossRef CAS; (f) B. R. Bear, S. M. Sparks and K. J. Shea, Angew. Chem., Int. Ed., 2001, 40, 820 CrossRef CAS; (g) E. Marsault, A. Toro, P. Nowak and P. Deslongchamps, Tetrahedron, 2001, 57, 4243 CrossRef CAS.
- For selected reviews, see: (a) C. S. Schindler and E. M. Carreira, Chem. Soc. Rev., 2009, 38, 3222 RSC; (b) K.-i. Takao, R. Munakata and K.-i. Tadano, Chem. Rev., 2005, 105, 4779 CrossRef CAS PubMed; (c) G. Brieger and J. N. Bennett, Chem. Rev., 1980, 80, 63 CrossRef CAS.
- (a) N. Krause and A. S. K. Hashmi, Modern Allene Chemistry, Wiley-VCH, Weinheim, 2004 Search PubMed; (b) T. Yoshino, F. Ng and S. J. Danishefsky, J. Am. Chem. Soc., 2006, 128, 14185 CrossRef CAS PubMed; (c) S. P. Cook, A. Polara and S. J. Danishefsky, J. Am. Chem. Soc., 2006, 128, 16440 CrossRef CAS PubMed; (d) M. Nendel, L. M. Tolbert, L. E. Herring, M. N. Islam and K. N. Houk, J. Org. Chem., 1999, 64, 976 CrossRef CAS PubMed; (e) H. V. Pham and K. N. Houk, J. Org. Chem., 2014, 79, 8968 CrossRef CAS PubMed; (f) M. E. Jung and N. Nishimura, Org. Lett., 2001, 3, 2113 CrossRef CAS PubMed; (g) J. K. Lam, Y. Schmidt and C. D. Vanderwal, Org. Lett., 2012, 14, 5566 CrossRef CAS PubMed.
- For selected examples, see: (a) B. Trillo, F. López, M. Gulías, L. Castedo and J. L. Mascareñas, Angew. Chem., Int. Ed., 2008, 47, 951 CrossRef CAS PubMed; (b) B. Trillo, F. López, S. Montserrat, G. Ujaque, L. Castedo, A. Lledós and J. L. Mascareñas, Chem. – Eur. J., 2009, 15, 3336 CrossRef CAS PubMed; (c) P. Mauleón, R. M. Zeldin, A. Z. González and F. D. Toste, J. Am. Chem. Soc., 2009, 131, 6348 CrossRef PubMed; (d) C. Nevado and A. M. Echavarren, Synthesis, 2005, 167 CAS; (e) A. Fürstner and P. W. Davies, Angew. Chem., Int. Ed., 2007, 46, 3410 CrossRef PubMed; (f) A. Arcadi, Chem. Rev., 2008, 108, 3266 CrossRef CAS PubMed; (g) H. C. Shen, Tetrahedron, 2008, 64, 3885 CrossRef CAS PubMed; (h) H. C. Shen, Tetrahedron, 2008, 64, 7847 CrossRef CAS PubMed; (i) I. Fernández and J. L. Mascareñas, Org. Biomol. Chem., 2012, 10, 699 RSC.
- (a) A. G. Lohse and R. P. Hsung, Org. Lett., 2009, 11, 3430 CrossRef CAS PubMed; (b) J. E. Antoline and R. P. Hsung, Synlett, 2008, 739 CAS; (c) L. F. You, R. P. Hsung, A. A. Bedermann, A. V. Kurdyumov, Y. Tang, G. S. Buchanan and K. P. Cole, Adv. Synth. Catal., 2008, 350, 2885 CrossRef CAS PubMed; (d) J. B. Feltenberger, R. Hayashi, Y. Tang, E. S. C. Babiash and R. P. Hsung, Org. Lett., 2009, 11, 3666 CrossRef CAS PubMed; (e) E. H. Krenske, K. N. Houk, A. G. Lohse, J. E. Antoline and R. P. Hsung, Chem. Sci., 2010, 1, 387 RSC; (f) R. Hayashi, J. B. Feltenberger and R. P. Hsung, Org. Lett., 2010, 12, 1152 CrossRef CAS PubMed; (g) A. G. Lohse, E. H. Krenske, J. E. Antoline, K. N. Houk and R. P. Hsung, Org. Lett., 2010, 12, 5506 CrossRef CAS PubMed; (h) A. G. Lohse and R. P. Hsung, Chem. – Eur. J., 2011, 17, 3812 CrossRef CAS PubMed; (i) J. E. Antoline, E. H. Krenske, A. G. Lohse, K. N. Houk and R. P. Hsung, J. Am. Chem. Soc., 2011, 133, 14443 CrossRef CAS PubMed; (j) A. G. Lohse, R. P. Hsung, M. D. Leider and S. K. Ghosh, J. Org. Chem., 2011, 76, 3246 CrossRef CAS PubMed; (k) J. B. Feltenberger and R. P. Hsung, Org. Lett., 2011, 13, 3114 CrossRef CAS PubMed; (l) H. Li, Y. Tang and R. P. Hsung, Tetrahedron Lett., 2012, 53, 6138 CrossRef CAS PubMed; (m) R. Hayashi, Z. X. Ma and R. P. Hsung, Org. Lett., 2012, 14, 252 CrossRef CAS PubMed; (n) E. H. Krenske, S. Z. He, J. Huang, Y. F. Du, K. N. Houk and R. P. Hsung, J. Am. Chem. Soc., 2013, 135, 5242 CrossRef CAS PubMed; (o) Y. F. Du, E. H. Krenske, J. E. Antoline, A. G. Lohse, K. N. Houk and R. P. Hsung, J. Org. Chem., 2013, 78, 1753 CrossRef CAS PubMed; (p) L. C. Fang, R. P. Hsung, Z. X. Ma and W. R. Presser, Org. Lett., 2013, 15, 4842 CrossRef CAS PubMed; (q) L. C. Fang and R. P. Hsung, Org. Lett., 2014, 16, 1826 CrossRef CAS PubMed; (r) S. Z. He, R. P. Hsung, W. R. Presser, Z. X. Ma and B. J. Haugen, Org. Lett., 2014, 16, 2180 CrossRef CAS PubMed.
- J. Hu, B. Tian, X.-Y. Wu and X.-F. Tong, Org. Lett., 2012, 14, 5074 CrossRef CAS PubMed.
- (a) E. A. Anderson, Org. Biomol. Chem., 2011, 9, 3997 RSC; (b) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134 CrossRef CAS PubMed; (c) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS PubMed.
- (a) J.-Q. Kuang and S.-M. Ma, J. Org. Chem., 2009, 74, 1763 CrossRef CAS PubMed; (b) J.-Q. Kuang and S.-M. Ma, J. Am. Chem. Soc., 2010, 132, 1786 CrossRef CAS PubMed.
- The crystal data of 2a have been deposited in the CCDC with number 999306.
- The crystal data of 3a have been deposited in the CCDC with number 995122.
- The low yield of this product may be due to the fact that it is quite volatile.
- The crystal data of 5a have been deposited in the CCDC with number 1009701.
- When using 4b as the substrate, we did not observe the targeted product under the standard conditions. Replacing dicyclohexylamine with tetrahydroisoquinoline afforded 7a in 55% isolated yield along with N-(2-(furan-2-yl)ethyl)-4-methylbenzenesulfonamide in 10% yield.
. - G. J. Jiang, Q. H. Zheng, M. Dou, L. G. Zhuo, W. Meng and Z. X. Yu, J. Org. Chem., 2013, 78, 11783 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data of new compounds. CCDC 999306 for 2a, 995122 for 3a, 1009701 for 5a. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00033e |
| This journal is © the Partner Organisations 2015 |