Indolizine synthesis via Cu-catalyzed cyclization of 2-(2-enynyl)pyridines with nucleophiles†
Ren-Rong
Liu
,
Zheng-Yi
Cai
,
Chuan-Jun
Lu
,
Shi-Chun
Ye
,
Bin
Xiang
,
Jianrong
Gao
and
Yi-Xia
Jia
*
College of Chemical Engineering, Zhejiang University of Technology, Chaowang Road 18#, Hangzhou 310014, China. E-mail: yxjia@zjut.edu.cn
First published on 14th January 2015
Abstract
Efficient and atom-economical access to indolizines has been developed by a Cu-catalyzed cyclization of 2-(2-enynyl)pyridines with various nucleophiles through simultaneous formation of a C–N bond and a remote carbon–nucleophile bond. 1,3-Dicarbonyl compounds, indoles, amides, alcohol, and even water, were used as nucleophiles in this reaction. Good to excellent yields of the corresponding indolizines were obtained under mild reaction conditions.
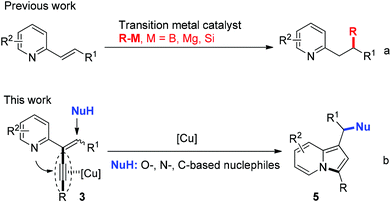
Indolizines are important building blocks in organic synthesis1 and unique N-fused heterocycles frequently found as backbones in many bioactive natural products and pharmaceuticals, such as anti-inflammatory agents, H3 receptor antagonists, and anti-HIV agents.2 Thus, the transformations based on readily available substrates to access diversely substituted indolizines are very important. So far, many methods have been documented for the synthesis of indolizines, including the Scholtz reaction,3 the Tschitschibabin reaction,4 the dipolar cycloadditions of pyridinium and related heteroaromatic ylides,5 the cyclization of pyridines with alkenyldiazoacetates,6 the C–H functionalization of indolizine,7etc.8 Most of the aforementioned approaches often involve multistage synthesis and limit substrate availability in some cases. Accordingly, the development of general and efficient synthetic methodology toward indolizine scaffolds is highly desirable.
Cycloisomerization transformation involving the activation of alkynes by transition metal catalysts is a very active area of current research.9 In this context, transformation of 2-propargylpyridine and 2-alkynylpyridine derivatives represent elegant and powerful approaches to indolizines through the triple bond activation and subsequent cycloisomerization in the presence of Pt, Au, Pd, or Cu-based catalysts.10,11 In recent years, the transition-metal catalyzed nucleophilic addition reactions of heteroaryl substituted olefins have been well studied. The nucleophiles applied in these reactions were limited to active organometallic reagents (such as organicboron reagents, Grignard reagents, and organosilicon reagents) (Scheme 1, eqn (a)), whereas the common N-, O-, and C-nucleophiles were not invovled.12 On the basis of these studies, we envisioned that the cyclization of 2-propargylpyridine involving alkyne activation would probably facilitate the nucleophilic additions of the common N-, O-, and C-nucleophiles to the alkene, which would provide a new way to the synthesis of indolizines. Herein, we communicate the synthesis of a designed substrate 2-(2-enynyl)pyridine and its cyclization reactions with various nucleophiles in the presence of a copper catalyst, affording structural diversely 1,3-disubstituted indolizines in good to excellent yields (Scheme 1, eqn (b)).13 We found that 1,3-dicarbonyl compounds, indoles, amides, alcohol, and even water, were suitable nucleophiles in this reaction. This process is quite attractive because the nucleophilic addition reaction and the cyclization occurred simultaneously to form a C–N bond and a remote carbon–nucleophile bond.
 | (1) |
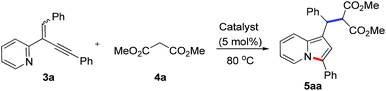
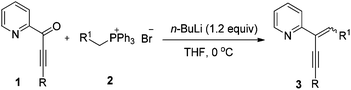
At the outset, the 2-(2-enynyl)pyridine substrates 3 were easily prepared by the Wittig reaction between pyridyl-substituted alkynyl ketones 1 and triphenylphosphonium salts 2 [eqn (1)].14 The cyclization reaction incorporating nucleophilic addition was then studied. 2-(1,4-Diphenylbut-1-en-3-yn-2-yl)pyridine 3a and dimethyl malonate 4a were chosen as model substrates and the reaction was performed using a series of potential transition metal catalysts (Table 1). Initial result indicated that the reaction proceeded smoothly in CH3CN using 5 mol% of CuI as a catalyst to afford the desired indolizine 5aa in 85% yield (entry 1). Pd(OAc)2, CuCl, CuBr, AgSbF6, and AuCl3 could also catalyze this reaction albeit with inferior yields (entries 2–6). However, no desired product was detected when PdCl2 was used as a catalyst (entry 7). The subsequent solvent examination showed that reduced yields were observed in dichloroethane and toluene (entries 8 and 9). To our delight, the desired product was isolated in 90% yield by introducing 1.0 equiv. of Et3N to the reaction (entry 10) and the yield was further improved to 98% when 4 Å molecular sieves was added (entry 11). Notably, significant lower yield was obtained when the reaction temperature was decreased to 50 °C (entry 12).
| Entry | Catalyst | Solvent | Additive | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 3a (0.2 mmol), 4a (1.05 equiv.), 5 mol% catalyst, 1.0 equiv. additive, with the solvent indicated (2.0 mL) at 80 °C. b Isolated yield. c 100 mg 4 Å molecular sieves was added. d Reaction was performed at 50 °C. | ||||
| 1 | CuI | CH3CN | — | 85 |
| 2 | Pd(OAc)2 | CH3CN | — | 80 |
| 3 | AuCl3 | CH3CN | — | 75 |
| 4 | CuBr | CH3CN | — | 79 |
| 5 | CuCl | CH3CN | — | 76 |
| 6 | AgSbF6 | CH3CN | — | 55 |
| 7 | PdCl2 | CH3CN | — | 0 |
| 8 | CuI | DCE | — | 73 |
| 9 | CuI | Toluene | — | 70 |
| 10 | CuI | CH3CN | Et3N | 90 |
| 11c | CuI | CH3CN | Et3N | 98 |
| 12d | CuI | CH3CN | Et3N | 50 |
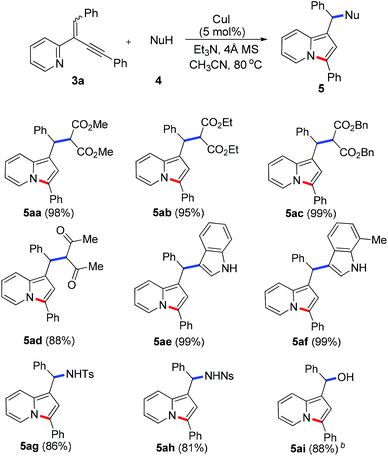
To demonstrate the generality of this reaction, a series of nucleophiles were then examined under the optimized conditions. As shown in Table 2, the reactions of 3a with various nucleophiles proceeded smoothly to afford the desired products in excellent yields. Different malonates reacted with 3a to give the corresponding indolizines 5aa–5ac in 95–99% yields. Acetylacetone was also a suitable C-based nucleophile to react with 3a, giving 5ad in 88% yield. A distinctive feature of this cyclization reaction is the facile introduction of electron-rich arenes (such as indoles) acting as C-based nucleophiles and amides acting as N-based nucleophiles, leading to indolizine-contained triarylmethanes (5ae and 5af) and diarylmethanamide (5ag and 5ah) in good to excellent yields. Moreover, as an O-based nucleophile, phenylmethanol coupled efficiently with 3a to afford diarylmethyl ether 5aj, which was unstable and was converted into ketone 6 in 85% yield in the presence of DDQ [eqn (2)]. In addition, water could also act as a suitable nucleophile to afford indolizine 5ai bearing a convertible alcohol in 88% yield.
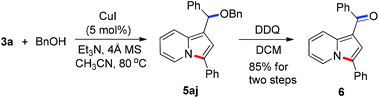 | (2) |
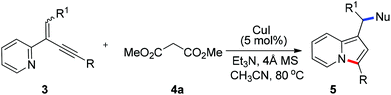
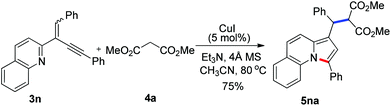
To further extend the substrate scope, we then turned our attention to the reactions of manolate 4a with a range of 2-(2-enynyl)pyridines under the optimized conditions and the results are summarized in Table 3. Excellent yields were obtained for the substrates containing two aryl substituents (5ba–5da, 5fa–5ja, and 5ma). However, relative lower yields were observed for those bearing alkyl groups (5ea and 5ka), especially when the alkyl group was linked to the alkene (5ka). The reaction of 2-quinolyl substrate 3n also proceeded smoothly to give benz[e]indolizine 5na in 75% yield [eqn (3)]. Moreover, an ester group linked to the alkene was also well tolerated and the reaction of 3l with 4a furnished product 5la in excellent yield.
| Entry | 3 (R/R1) | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 3a (0.2 mmol), 4a (1.05 equiv.), 5 mol% CuI, 1.0 equiv. Et3N, and 4 Å molecular sieves (100 mg) in CH3CN (2.0 mL) at 80 °C. b Isolated yield. c 1.5 equiv. 4a was used. | ||
| 1 | 3b (p-Me-C6H4/Ph) | 5ba (99) |
| 2 | 3c (p-Me-C6H4/Ph) | 5ca (98) |
| 3 | 3d (p-OMe-C6H4/Ph) | 5da (98) |
| 4 | 3e (n-C4H9/Ph) | 5ea (80) |
| 5 | 3f (Ph/p-F-C6H4) | 5fa (99) |
| 6 | 3g (Ph/p-Me-C6H4) | 5ga (98) |
| 7 | 3h (Ph/m-F-C6H4) | 5ha (99) |
| 8 | 3i (Ph/m-Me-C6H4) | 5ia (98) |
| 9 | 3j (Ph/o-Br-C6H4) | 5ja (98) |
| 10c | 3k (Ph/n-C3H7) | 5ka (65) |
| 11 | 3l (Ph/CO2Me) | 5la (95) |
| 12 | 3m (p-OMe-C6H4/p-Me-C6H4) | 5ma (95) |
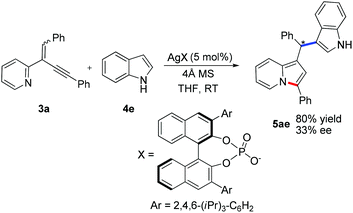
Preliminary results shown in Scheme 2 indicated that this reaction was amendable to enantioselective catalysis albeit the ee value was unsatisfactory at this stage. Under the catalysis of a chiral silver phosphate complex, the reaction of (1,4-diphenylbut-1-en-3-yn-2-yl)pyridine 3a with indole 4e proceeded smoothly in THF to afford the indolizine 5ae in 80% yield and with 33% ee (Scheme 2).15
 | (3) |
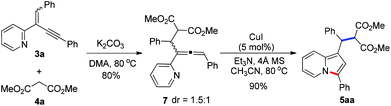
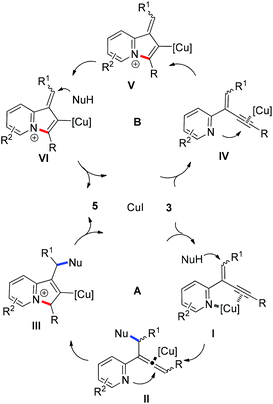
To get insight into the mechanism for this CuI-catalyzed cyclization reaction, a brief preliminary experiment was carried out. As shown in Scheme 3, allenylpyridine 7 was prepared in 80% yield by the addition of methyl malonate 4a to 2-(2-enynyl)pyridine 3a promoted by K2CO3 in the absence of CuI.16 When compound 7 was subjected to the reaction conditions, indolizine 5aa was obtained in 90% yield. This result implies that the reaction might involve such an allenylpyridine intermediate. As depicted in catalytic cycle A (Fig. 1), CuI functions as both a Lewis acid and a transition metal to coordinate with pyridine and alkyne, which increases the electrophilicity of the alkene and thereby promotes the addition of the nucleophile to the alkene and generates the allenylpyridine intermediate II. This intermediate then undergoes in situ cyclization in the presence of Cu(I) salt to produce an indolizine. However, we cannot rule out an alternative pathway shown in cycle B, where CuI functions as a transition metal to activate the alkyne and facilitates the intramolecular nucleophilic attack of pyridine to alkynes. The resulting intermediate V is then trapped by nucleophiles, followed by protonation and subsequent isomerization, to produce an indolizine and regenerate the Cu(I) catalyst.
Conclusions
In summary, we have developed an efficient and atom-economical route to 1,3-substituted indolizines in good to excellent yields through a CuI-catalyzed cyclization of 2-(2-enynyl)pyridine with various nucleophiles under mild reaction conditions. 1,3-Dicarbonyl compounds, indoles, amides, alcohol, and even water, were involved in this reaction as nucleophiles. The substrate 2-(2-enynyl)pyridines could be easily prepared from pyridyl-substituted alkynyl ketones by the Wittig reaction, which makes this process more practical and useful.The project was supported by the National Natural Science Foundation of China (grant no. 21002089; 21372202), New Century Excellent Talents in University (NCET-12-1086), and Zhejiang Natural Science Fund for Distinguished Young Scholars (R14B020005).
Notes and references
- For reviews, see: (a) J. P. Micheal, Alkaloids, 2001, 55, 91 Search PubMed; (b) J. P. Micheal, Nat. Prod. Rep., 2002, 19, 742 RSC; (c) T. Uchida and K. Matsumoto, Synthesis, 1976, 209 CrossRef CAS; (d) A. Behnisch, P. Behnisch, M. Eggenweiler and T. Wallenhorst, Indolizine, in Houben-Weyl, Thieme, Stuttgart, Germany, 1994, vol. E6b/1, 2a, p. 323 Search PubMed.
- (a) R. J. Molyneux and L. F. James, Science, 1982, 216, 190 CAS; (b) R. C. Oslund, N. Cermak and M. H. Gelb, J. Med. Chem., 2008, 51, 4708 CrossRef CAS PubMed; (c) S. P. Gupta, A. N. Mathur, A. N. Nagappa, D. Kumar and S. Kumaran, Eur. J. Med. Chem., 2003, 38, 867 CrossRef CAS PubMed; (d) M. Facompre, C. Tardy, C. Bal-Mahieu, P. Colson, C. Perez, I. Manzanares, C. Cuevas and C. Bailly, Cancer Res., 2003, 63, 7392 CAS; (e) E. Kim, M. Koh, J. Ryu and S. B. Park, J. Am. Chem. Soc., 2008, 130, 12206 CrossRef CAS PubMed.
- V. Boekelheide and R. J. Windgassen, J. Am. Chem. Soc., 1959, 81, 1456 CrossRef CAS.
- (a) J. Hurst, T. Melton and D. G. Wibberley, J. Chem. Soc., 1965, 2948 RSC; (b) U. Bora, A. Saikia and R. C. Boruah, Org. Lett., 2003, 5, 43 CrossRef PubMed.
- (a) G. Poissonnet, M.-H. Theret-Bettiol and R. H. Dodd, J. Org. Chem., 1996, 61, 2273 CrossRef CAS; (b) A. R. Katritzky, G. Qiu, B. Yang and H.-Y. He, J. Org. Chem., 1999, 64, 7618 CrossRef CAS.
- J. Barluenga, G. Lonzi, L. Riesgo, L. A. Lopez and M. Tomas, J. Am. Chem. Soc., 2010, 132, 13200 CrossRef CAS PubMed.
- (a) J.-B. Xia and S.-L. You, Org. Lett., 2009, 11, 1187 CrossRef CAS PubMed; (b) Y. Yang, C. Xie, Y. Xie and Y. Zhang, Org. Lett., 2012, 14, 957 CrossRef CAS PubMed; (c) X. Wang, S.-Y. Li, Y.-M. Pan, H. Wang, H. Liang, Z. Chen and X. Qin, Org. Lett., 2014, 16, 580 CrossRef CAS PubMed; (d) Y. Yang, K. Cheng and Y. Zhang, Org. Lett., 2009, 11, 5606 CrossRef CAS PubMed.
- (a) J. Kaloko and A. Hayford, Org. Lett., 2005, 7, 4305 CrossRef CAS PubMed; (b) H. Kim, K. Lee, S. Kim and P. Ho Lee, Chem. Commun., 2010, 46, 6341 RSC; (c) H. Zhu, J. Stoockigt, Y. Yu and H. Zou, Org. Lett., 2011, 13, 2792 CrossRef CAS PubMed; (d) Q. Wu, D. Zhao, X. Qin, J. Lan and J. You, Chem. Commun., 2011, 47, 9188 RSC; (e) M. Kim, Y. Jung and I. Kim, J. Org. Chem., 2013, 78, 10395 CrossRef CAS PubMed; (f) X. Meng, P. Liao, J. Liua and X. Bi, Chem. Commun., 2014, 50, 11837 RSC; (g) J. Liu, L. Zhou, W. Ye and C. Wang, Chem. Commun., 2014, 50, 9068 RSC; (h) L. H. Phun, J. Aponte-Guzman and S. France, Angew. Chem., Int. Ed., 2012, 51, 3198 CrossRef CAS PubMed; (i) E. J. Choi, E. Kim, Y. Lee, A. Jo and S. B. Park, Angew. Chem., Int. Ed., 2014, 53, 1346 CrossRef CAS PubMed.
- For reviews see: (a) A. V. Gulevich, A. S. Dudnik, N. Chernyak and V. Gevorgyan, Chem. Rev., 2013, 113, 3084 CrossRef CAS PubMed; (b) B. Chattopadhyay and V. Gevorgyan, Angew. Chem., Int. Ed., 2012, 51, 862 CrossRef CAS PubMed; (c) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS PubMed.
- (a) I. V. Seregin and V. Gevorgyan, J. Am. Chem. Soc., 2006, 128, 12050 CrossRef CAS PubMed; (b) I. V. Seregin, A. W. Schammel and V. Gevorgyan, Org. Lett., 2007, 9, 3433 CrossRef CAS PubMed; (c) B. Yan, Y. Zhou, H. Zhang, J. Chen and Y. Liu, J. Org. Chem., 2007, 72, 7783 CrossRef CAS PubMed; (d) B. Yan and Y. Liu, Org. Lett., 2007, 9, 4323 CrossRef CAS PubMed; (e) C. R. Smith, E. M. Bunnelle, A. J. Rhodes and R. Sarpong, Org. Lett., 2007, 9, 1169 CrossRef CAS PubMed.
- (a) A. V. Kel'in, A. W. Sromek and V. Gevorgyan, J. Am. Chem. Soc., 2001, 123, 2074 CrossRef; (b) Y. Liu, Z. Song and B. Yan, Org. Lett., 2007, 9, 409 CrossRef CAS PubMed.
- (a) M. Lautens, A. Roy, K. Fukuoka, K. Fagnou and B. Martin-Matute, J. Am. Chem. Soc., 2001, 123, 5358 CrossRef CAS; (b) L. Rupnicki, A. Saxena and H. W. Lam, J. Am. Chem. Soc., 2009, 131, 10386 CrossRef CAS PubMed; (c) G. Pattison, G. Piraux and H. W. Lam, J. Am. Chem. Soc., 2010, 132, 14373 CrossRef CAS PubMed; (d) I. D. Roy, A. R. Burns, G. Pattison, B. Michel, A. G. Parkerc and H. W. Lam, Chem. Commun., 2014, 50, 2865 RSC; (e) H. Gilman and G. C. Gainer, J. Am. Chem. Soc., 1949, 71, 2327 CrossRef CAS.
- For the reactions of 2-(1-alkynyl)-2-alken-1-one, see: (a) T. Yao, X. Zhang and R. C. Larock, J. Am. Chem. Soc., 2004, 126, 11164 CrossRef CAS PubMed; (b) N. T. Patil, H. Wu and Y. Yamamoto, J. Org. Chem., 2005, 70, 4531 CrossRef CAS PubMed; (c) Y. Liu and S. Zhou, Org. Lett., 2005, 7, 4609 CrossRef CAS PubMed; (d) Y. Xiao and J. Zhang, Angew. Chem., Int. Ed., 2008, 47, 1903 CrossRef CAS PubMed; (e) F. Liu, Y. Yu and J. Zhang, Angew. Chem., Int. Ed., 2009, 48, 5505 CrossRef CAS PubMed; (f) G. Zhou and J. Zhang, Chem. Commun., 2010, 46, 6593 RSC; (g) W. Li and J. Zhang, Chem. Commun., 2010, 46, 8839 RSC; (h) L. Zhou, M. Zhang, W. Li and J. Zhang, Angew. Chem., Int. Ed., 2014, 53, 6542 CrossRef CAS PubMed; (i) W. Zhao and J. Zhang, Org. Lett., 2011, 13, 688 CrossRef CAS PubMed; (j) X. Yu and J. Zhang, Chem. – Eur. J., 2012, 18, 12945 CrossRef CAS PubMed; (k) X. Yu and J. Zhang, Chem. – Eur. J., 2008, 14, 8481 CrossRef CAS PubMed; (l) L. Zhou, M. Zhang, W. Li and J. Zhang, Angew. Chem., Int. Ed., 2014, 53, 6542 CrossRef CAS PubMed.
- Please see the detail preparing methods in ESI.†.
- For selected examples of chiral-anion catalysis by chiral phosphates in transition metal-catalyzed reactions, see: (a) G. L. Hamilton, E. J. Kang, M. Mba and F. D. Toste, Science, 2007, 317, 496 CrossRef CAS PubMed; (b) S. Mukherjee and B. List, J. Am. Chem. Soc., 2007, 129, 11336 CrossRef CAS PubMed; (c) M. Rueping, A. P. Antonchick and C. Brinkmann, Angew. Chem., Int. Ed., 2007, 46, 6903 CrossRef CAS PubMed; (d) Z.-L. Tao, W.-Q. Zhang, D.-F. Chen, A. Adele and L.-Z. Gong, J. Am. Chem. Soc., 2013, 135, 9255 CrossRef CAS PubMed; (e) M. Terada, F. Li and Y. Toda, Angew. Chem., Int. Ed., 2014, 53, 235 CrossRef CAS PubMed; (f) V. Rauniyar, Z. J. Wang, H. E. Burks and F. D. Toste, J. Am. Chem. Soc., 2011, 133, 8486 CrossRef CAS PubMed.
- For allene synthesis reviews, please see: (a) S. Ma, Chem. Rev., 2005, 105, 2829 CrossRef PubMed; (b) S. Ma, Aldrichimica Acta, 2005, 40, 91 Search PubMed; (c) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994 CrossRef CAS PubMed. For selected examples please see: (d) J. Kuang and S. Ma, J. Org. Chem., 2009, 74, 1763 CrossRef CAS PubMed; (e) H. Jiang, X. Liu and L. Zhou, Chem. – Eur. J., 2008, 14, 11305 CrossRef CAS PubMed; (f) C. Y. Li, X. B. Wang, X. L. Sun, Y. Tang, J. C. Zheng, Z. H. Xu, Y. G. Zhou and L. X. Dai, J. Am. Chem. Soc., 2007, 129, 1494 CrossRef CAS PubMed; (g) Y. Xiao and J. Zhang, Chem. Commun., 2010, 46, 752 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation of substrates, characterization data, 1H, 13C NMR, HRMS. See DOI: 10.1039/c4qo00336e |
| This journal is © the Partner Organisations 2015 |